Microarray scanner calibration curves: characteristics and implications
- PMID: 16026596
- PMCID: PMC1637029
- DOI: 10.1186/1471-2105-6-S2-S11
Microarray scanner calibration curves: characteristics and implications
Abstract
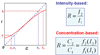
Background: Microarray-based measurement of mRNA abundance assumes a linear relationship between the fluorescence intensity and the dye concentration. In reality, however, the calibration curve can be nonlinear.
Results: By scanning a microarray scanner calibration slide containing known concentrations of fluorescent dyes under 18 PMT gains, we were able to evaluate the differences in calibration characteristics of Cy5 and Cy3. First, the calibration curve for the same dye under the same PMT gain is nonlinear at both the high and low intensity ends. Second, the degree of nonlinearity of the calibration curve depends on the PMT gain. Third, the two PMTs (for Cy5 and Cy3) behave differently even under the same gain. Fourth, the background intensity for the Cy3 channel is higher than that for the Cy5 channel. The impact of such characteristics on the accuracy and reproducibility of measured mRNA abundance and the calculated ratios was demonstrated. Combined with simulation results, we provided explanations to the existence of ratio underestimation, intensity-dependence of ratio bias, and anti-correlation of ratios in dye-swap replicates. We further demonstrated that although Lowess normalization effectively eliminates the intensity-dependence of ratio bias, the systematic deviation from true ratios largely remained. A method of calculating ratios based on concentrations estimated from the calibration curves was proposed for correcting ratio bias.
Conclusion: It is preferable to scan microarray slides at fixed, optimal gain settings under which the linearity between concentration and intensity is maximized. Although normalization methods improve reproducibility of microarray measurements, they appear less effective in improving accuracy.
Figures









References
-
- Pickett S. Understanding and evaluating fluorescent microarray imaging instruments. IVT Technology. 2003;9:1–6.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

