A malarial cysteine protease is necessary for Plasmodium sporozoite egress from oocysts
- PMID: 16027235
- PMCID: PMC2213010
- DOI: 10.1084/jem.20050545
A malarial cysteine protease is necessary for Plasmodium sporozoite egress from oocysts
Abstract
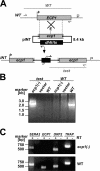
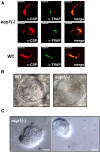
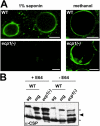
The Plasmodium life cycle is a sequence of alternating invasive and replicative stages within the vertebrate and invertebrate hosts. How malarial parasites exit their host cells after completion of reproduction remains largely unsolved. Inhibitor studies indicated a role of Plasmodium cysteine proteases in merozoite release from host erythrocytes. To validate a vital function of malarial cysteine proteases in active parasite egress, we searched for target genes that can be analyzed functionally by reverse genetics. Herein, we describe a complete arrest of Plasmodium sporozoite egress from Anopheles midgut oocysts by targeted disruption of a stage-specific cysteine protease. Our findings show that sporozoites exit oocysts by parasite-dependent proteolysis rather than by passive oocyst rupture resulting from parasite growth. We provide genetic proof that malarial cysteine proteases are necessary for egress of invasive stages from their intracellular compartment and propose that similar cysteine protease-dependent mechanisms occur during egress from liver-stage and blood-stage schizonts.
Figures

References
-
- Sibley, L.D. 2004. Intracellular parasite invasion strategies. Science. 304:248–253. - PubMed
-
- Blackman, M.J. 2000. Proteases involved in erythrocyte invasion by the malaria parasite: function and potential as chemotherapeutic targets. Curr. Drug Targets. 1:59–83. - PubMed
-
- Hadley, T., M. Aikawa, and L.H. Miller. 1983. Plasmodium knowlesi: studies on invasion of rhesus erythrocytes by merozoites in the presence of protease inhibitors. Exp. Parasitol. 55:306–311. - PubMed
-
- Lyon, J.A., and J.D. Haynes. 1986. Plasmodium falciparum antigens synthesized by schizonts and stabilized at the merozoite surface when schizonts mature in the presence of protease inhibitors. J. Immunol. 136:2245–2251. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

