Toll-like receptor 9 controls anti-DNA autoantibody production in murine lupus
- PMID: 16027240
- PMCID: PMC2212997
- DOI: 10.1084/jem.20050338
Toll-like receptor 9 controls anti-DNA autoantibody production in murine lupus
Abstract
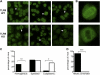
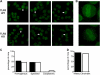
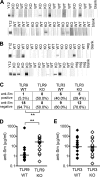
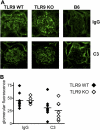
Systemic autoimmune disease in humans and mice is characterized by loss of immunologic tolerance to a restricted set of self-nuclear antigens. Autoantigens, such as double-stranded (ds) DNA and the RNA-containing Smith antigen (Sm), may be selectively targeted in systemic lupus erythematosus because of their ability to activate a putative common receptor. Toll-like receptor 9 (TLR9), a receptor for CpG DNA, has been implicated in the activation of autoreactive B cells in vitro, but its role in promoting autoantibody production and disease in vivo has not been determined. We show that in TLR9-deficient lupus-prone mice, the generation of anti-dsDNA and antichromatin autoantibodies is specifically inhibited. Other autoantibodies, such as anti-Sm, are maintained and even increased in TLR9-deficient mice. In contrast, ablation of TLR3, a receptor for dsRNA, did not inhibit the formation of autoantibodies to either RNA- or DNA-containing antigens. Surprisingly, we found that despite the lack of anti-dsDNA autoantibodies in TLR9-deficient mice, there was no effect on the development of clinical autoimmune disease or nephritis. These results demonstrate a specific requirement for TLR9 in autoantibody formation in vivo and indicate a critical role for innate immune activation in autoimmunity.
Figures



References
-
- Notman, D.D., N. Kurata, and E.M. Tan. 1975. Profiles of antinuclear antibodies in systemic rheumatic diseases. Ann. Intern. Med. 83:464–469. - PubMed
-
- Kotzin, B. 1996. Systemic lupus erythematosus. Cell. 85:303–306. - PubMed
-
- Plotz, P.H. 2003. The autoantibody repertoire: searching for order. Nat. Rev. Immunol. 3:73–78. - PubMed
-
- Monestier, M., and B.L. Kotzin. 1992. Antibodies to histones in systemic lupus erythematosus and drug-induced lupus syndromes. Rheum. Dis. Clin. North Am. 18:415–436. - PubMed
-
- Hahn, B.H. 1998. Antibodies to DNA. N. Engl. J. Med. 338:1359–1368. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

