Expansion of calcium microdomains regulates fast exocytosis at a ribbon synapse
- PMID: 16027365
- PMCID: PMC1180766
- DOI: 10.1073/pnas.0501961102
Expansion of calcium microdomains regulates fast exocytosis at a ribbon synapse
Abstract
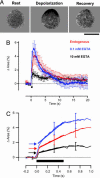
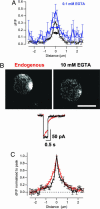
We investigated the Ca2+ signal regulating fast exocytosis at the ribbon synapse of retinal bipolar cells by using total internal reflection fluorescence microscopy to image fluorescent Ca2+ indicators and interference reflection microscopy to monitor exocytosis. Depolarization generated Ca2+ "microdomains" that expanded over the time scale during which the rapidly releasable pool (RRP) of vesicles was released (<40 ms). Replacing mobile Ca2+ buffers in the terminal with 10 mM EGTA prevented expansion of microdomains and decreased the number of rapidly releasable vesicles by a factor of 2. Conversely, decreasing the concentration of EGTA in the terminal to 0.1 mM increased the apparent width of a Ca2+ microdomain from 580 nm to 930 nm and increased the size of the RRP size by a factor of 1.5. The [Ca2+] over the area that the microdomain expanded was estimated to be 2-7 microM. These results indicate that vesicles within the RRP are located hundreds of nanometers from Ca2+ channels, and that fusion of these vesicles can be triggered by low micromolar levels of Ca2+. Variable distances between docked vesicles and Ca2+ channels at the active zone, therefore, provide an explanation for the heterogeneous release probability of vesicles comprising the RRP.
Figures



References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

