Two-dimensional atomic crystals
- PMID: 16027370
- PMCID: PMC1180777
- DOI: 10.1073/pnas.0502848102
Two-dimensional atomic crystals
Abstract
We report free-standing atomic crystals that are strictly 2D and can be viewed as individual atomic planes pulled out of bulk crystals or as unrolled single-wall nanotubes. By using micromechanical cleavage, we have prepared and studied a variety of 2D crystals including single layers of boron nitride, graphite, several dichalcogenides, and complex oxides. These atomically thin sheets (essentially gigantic 2D molecules unprotected from the immediate environment) are stable under ambient conditions, exhibit high crystal quality, and are continuous on a macroscopic scale.
Figures


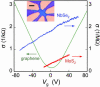
References
-
- Kroto, H. W., Fischer, J. E. & Cox, D. E., eds. (1993) The Fullerenes (Pergamon, Oxford).
-
- Iijima, S. (1991) Nature 354, 56-58.
-
- Chopra, N. G., Luyken, R. J., Cherrey, K., Crespi, V. H., Cohen, M. L., Louie, S. G. & Zettl, A. (1995) Science 269, 966-967. - PubMed
-
- Tenne, R., Margulis, L., Genut, M. & Hodes, G. (1992) Nature 360, 444-446.
-
- Dresselhaus, M. S. & Dresselhaus, G. (2002) Adv. Phys. 51, 1-186.
LinkOut - more resources
Full Text Sources
Other Literature Sources

