Exploiting the Pd- and Ru-catalyzed cycloisomerizations: enantioselective total synthesis of (+)-allocyathin B2
- PMID: 16028937
- PMCID: PMC2565572
- DOI: 10.1021/ja051547m
Exploiting the Pd- and Ru-catalyzed cycloisomerizations: enantioselective total synthesis of (+)-allocyathin B2
Abstract
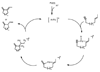
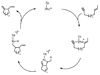
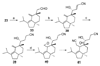
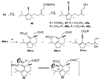
Pd- and Ru-catalyzed cycloisomerizations of 1,6-enynes are compared and contrasted. Such considerations led to the enantioselective synthesis of a cyathin terpenoid, (+)-allocyathin B(2) (1). The synthesis features a Pd-catalyzed asymmetric allylic alkylation (AAA) to install the initial quaternary center, a Ru-catalyzed diastereoselective cycloisomerization to construct the six-membered ring, and a diastereoselective hydroxylative Knoevenagel reaction to introduce the final hydroxyl group. We demonstrate for the first time a mechanism-based stereochemical divergence in Pd- and Ru-catalyzed cycloisomerization reactions as well as in creation of alkene geometry with alkynes bearing a carboalkoxy group. Mechanistic rationalization is proposed for these observations.
Figures












References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

