Dopamine depletion alters phosphorylation of striatal proteins in a model of Parkinsonism
- PMID: 16029214
- PMCID: PMC4819997
- DOI: 10.1111/j.1460-9568.2005.04190.x
Dopamine depletion alters phosphorylation of striatal proteins in a model of Parkinsonism
Abstract
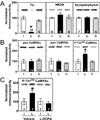
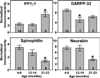
Nigrostriatal dopamine depletion disrupts striatal medium spiny neuron morphology in Parkinson's disease and modulates striatal synaptic plasticity in animal models of parkinsonism. We demonstrate that long-term nigrostriatal dopamine depletion in the rat induces evolving changes in the phosphorylation of striatal proteins critical for synaptic plasticity. Dopamine depletion increased the phosphorylation of the alpha isoform of calcium-calmodulin-dependent protein kinase II (CaMKIIalpha) at Thr286, a site associated with enhanced autonomous kinase activity, but did not alter total levels of CaMKIIalpha or other synaptic proteins. Dopamine depletion decreased CaMKIIalpha levels in postsynaptic density-enriched fractions without significant changes in other proteins. The activity of protein phosphatase 1 (PP1), a postsynaptic phosphatase that dephosphorylates CaMKII, is regulated by DARPP-32 (dopamine- and cAMP-regulated phosphoprotein of 32 kDa). Dopamine depletion had no effect on DARPP-32 phosphorylation at Thr34, but increased DARPP-32 phosphorylation at Thr75. Levodopa administration reversed the increased phosphorylation of both CaMKIIalpha and DARPP-32. Normal ageing increased the levels of PP1(gamma1 isoform) but decreased levels of the PP1gamma1-targeting proteins spinophilin and neurabin. Elevated phosphorylations of CaMKIIalpha and DARPP-32 were maintained for up to 20 months after dopamine depletion. However, phosphorylation of the CaMKII-PP1 substrate, Ser831 in the glutamate receptor GluR1 subunit, was increased only after sustained (9-20 months) dopamine depletion. Interaction of ageing-related changes in PP1 with the dopamine depletion-induced changes in CaMKIIalpha may account for enhanced GluR1 phosphorylation only after long-term dopamine depletion. These evolving changes may impact striatal synaptic plasticity, Parkinson's disease progression and the changing efficacy and side-effects associated with dopamine replacement therapy.
Figures




References
-
- Allen PB. Functional plasticity in the organization of signaling complexes in the striatum. Parkinsonism Related Disorders. 2004;10:287–292. - PubMed
-
- Betarbet R, Poisik O, Sherer TB, Greenamyre JT. Differential expression and ser897 phosphorylation of striatal N-methyl-D-aspartate receptor subunit NR1 in animal models of Parkinson’s disease. Exp. Neurol. 2004;187:76–85. - PubMed
-
- Carmody LC, Bauman PA, Bass MA, Mavila N, DePaoli-Roach AA, Colbran RJ. A protein phosphatase-1gamma1 isoform selectivity determinant in dendritic spine-associated neurabin. J. Biol. Chem. 2004;279:21714–21723. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

