Overexpression of Shp2 tyrosine phosphatase is implicated in leukemogenesis in adult human leukemia
- PMID: 16030196
- PMCID: PMC1895328
- DOI: 10.1182/blood-2004-10-4057
Overexpression of Shp2 tyrosine phosphatase is implicated in leukemogenesis in adult human leukemia
Abstract
Shp2 tyrosine phosphatase plays a critical role in hematopoiesis, and dominant active mutations have been detected in the human gene PTPN11, encoding Shp2, in child leukemia patients. We report here that although no such mutations were detected in 44 adult leukemia patients screened, Shp2 expression levels were significantly elevated in primary leukemia cells and leukemia cell lines, as compared with normal hematopoietic progenitor cells. The Shp2 protein amounts correlated well with the hyperproliferative capacity but were inversely associated with the differentiation degree of leukemia cells. Suppression of Shp2 expression induced apoptosis and inhibition of leukemic cell clonogenic growth. Notably, the majority of Shp2 was preferentially localized to the plasma membrane and was constitutively phosphorylated on tyrosine in leukemia cells, and also in normal hematopoietic cells following mitogenic stimulation. Based on these results, we propose that aberrantly increased expression of Shp2 may contribute, collaboratively with other factors, to leukemogenesis.
Figures



 ) versus Shp2 (□) contents in samples enriched with resting or proliferating hematopoietic cells. Data are representative of 3 independent experiments.
) versus Shp2 (□) contents in samples enriched with resting or proliferating hematopoietic cells. Data are representative of 3 independent experiments.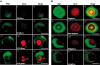



References
-
- Chan RJ, Johnson SA, Li Y, Yoder MC, Feng GS. A definitive role of Shp-2 tyrosine phosphatase in mediating embryonic stem cell differentiation and hematopoiesis. Blood. 2003;102: 2074-2080. - PubMed
-
- Qu CK, Nguyen S, Chen J, Feng GS. Requirement of Shp-2 tyrosine phosphatase in lymphoid and hematopoietic cell development. Blood. 2001;97: 911-914. - PubMed
-
- Boulton TG, Stahl N, Yancopoulos GD. Ciliary neurotrophic factor/leukemia inhibitory factor/interleukin 6/oncostatin M family of cytokines induces tyrosine phosphorylation of a common set of proteins overlapping those induced by other cytokines and growth factors. J Biol Chem. 1994;269: 11648-11655. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

