The stringent response is required for amino acid and nitrate utilization, nod factor regulation, nodulation, and nitrogen fixation in Rhizobium etli
- PMID: 16030199
- PMCID: PMC1196017
- DOI: 10.1128/JB.187.15.5075-5083.2005
The stringent response is required for amino acid and nitrate utilization, nod factor regulation, nodulation, and nitrogen fixation in Rhizobium etli
Abstract
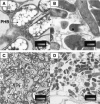
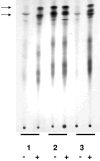
A Rhizobium etli Tn5 insertion mutant, LM01, was selected for its inability to use glutamine as the sole carbon and nitrogen source. The Tn5 insertion in LM01 was localized to the rsh gene, which encodes a member of the RelA/SpoT family of proteins. The LM01 mutant was affected in the ability to use amino acids and nitrate as nitrogen sources and was unable to accumulate (p)ppGpp when grown under carbon and nitrogen starvation, as opposed to the wild-type strain, which accumulated (p)ppGpp under these conditions. The R. etli rsh gene was found to restore (p)ppGpp accumulation to a DeltarelA DeltaspoT mutant of Escherichia coli. The R. etli Rsh protein consists of 744 amino acids, and the Tn5 insertion in LM01 results in the synthesis of a truncated protein of 329 amino acids; complementation experiments indicate that this truncated protein is still capable of (p)ppGpp hydrolysis. A second rsh mutant of R. etli, strain AC1, was constructed by inserting an Omega element at the beginning of the rsh gene, resulting in a null allele. Both AC1 and LM01 were affected in Nod factor production, which was constitutive in both strains, and in nodulation; nodules produced by the rsh mutants in Phaseolus vulgaris were smaller than those produced by the wild-type strain and did not fix nitrogen. In addition, electron microscopy revealed that the mutant bacteroids lacked poly-beta-hydroxybutyrate granules. These results indicate a central role for the stringent response in symbiosis.
Figures




References
-
- Aravind, L., and E. V. Koonin. 1998. The HD domain defines a new superfamily of metal-dependent phosphohydrolases. Trends Biochem. Sci. 23:469-472. - PubMed
-
- Belitsky, B., and C. Kari. 1982. Absence of accumulation of ppGpp and RNA during amino acid starvation in Rhizobium meliloti. J. Biol. Chem. 257:4677-4679. - PubMed
-
- Boyer, H. W., and D. Roulland-Dussoix. 1969. A complementation analysis of the restriction and modification of the DNA in Escherichia coli. J. Mol. Biol. 41:459-472. - PubMed
-
- Cashel, M. 1994. Detection of (p)ppGpp accumulation patterns in Escherichia coli mutants. Methods Mol. Genet. 3:341-356.
-
- Cashel, M., D. R. Gentry, V. J. Hernandez, and D. Vinella. 1996. The stringent response, p. 1458-1495. In F. C. Neidhart, R. Curtiss III, J. L. Ingraham, E. C. C. Lin, K. B. Low, B. Magasanik, K. W. S. Reznikoff, M. Riley, M. Schaechter, and H. E. Umbarger (ed.), Escherichia coli and Salmonella: cellular and molecular biology, 2nd ed., vol. I. ASM Press, Washington, D.C.
Publication types
MeSH terms
Substances
Associated data
- Actions
LinkOut - more resources
Full Text Sources

