Cystic fibrosis sputum supports growth and cues key aspects of Pseudomonas aeruginosa physiology
- PMID: 16030221
- PMCID: PMC1196007
- DOI: 10.1128/JB.187.15.5267-5277.2005
Cystic fibrosis sputum supports growth and cues key aspects of Pseudomonas aeruginosa physiology
Abstract
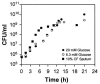
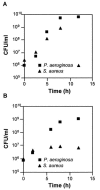
The opportunistic human pathogen Pseudomonas aeruginosa causes persistent airway infections in patients with cystic fibrosis (CF). To establish these chronic infections, P. aeruginosa must grow and proliferate within the highly viscous sputum in the lungs of CF patients. In this study, we used Affymetrix GeneChip microarrays to investigate the physiology of P. aeruginosa grown using CF sputum as the sole source of carbon and energy. Our results indicate that CF sputum readily supports high-density P. aeruginosa growth. Furthermore, multiple signals, which reduce swimming motility and prematurely activate the Pseudomonas quinolone signal cell-to-cell signaling cascade in P. aeruginosa, are present in CF sputum. P. aeruginosa factors critical for lysis of the common CF lung inhabitant Staphylococcus aureus were also induced in CF sputum and increased the competitiveness of P. aeruginosa during polymicrobial growth in CF sputum.
Figures
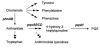



References
-
- Barth, A. L., and T. L. Pitt. 1996. The high amino-acid content of sputum from cystic fibrosis patients promotes growth of auxotrophic Pseudomonas aeruginosa. J. Med. Microbiol. 45:110-119. - PubMed
-
- Burns, J. L., B. W. Ramsey, and A. L. Smith. 1993. Clinical manifestations and treatment of pulmonary infections in cystic fibrosis. Adv. Pediatr. Infect. Dis. 8:53-66. - PubMed
-
- Collier, D. N., L. Anderson, S. L. McKnight, T. L. Noah, M. Knowles, R. Boucher, U. Schwab, P. Gilligan, and E. C. Pesci. 2002. A bacterial cell to cell signal in the lungs of cystic fibrosis patients. FEMS Microbiol. Lett. 215:41-46. - PubMed
-
- Costerton, J. W., Z. Lewandowski, D. E. Caldwell, D. R. Korber, and H. M. Lappin-Scott. 1995. Microbial biofilms. Annu. Rev. Microbiol. 49:711-745. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

