Molecular interactions position Mso1p, a novel PTB domain homologue, in the interface of the exocyst complex and the exocytic SNARE machinery in yeast
- PMID: 16030256
- PMCID: PMC1237063
- DOI: 10.1091/mbc.e05-03-0243
Molecular interactions position Mso1p, a novel PTB domain homologue, in the interface of the exocyst complex and the exocytic SNARE machinery in yeast
Abstract
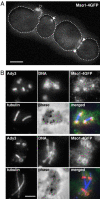
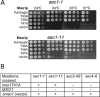
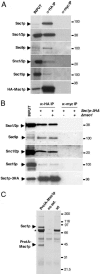
In this study, we have analyzed the association of the Sec1p interacting protein Mso1p with the membrane fusion machinery in yeast. We show that Mso1p is essential for vesicle fusion during prospore membrane formation. Green fluorescent protein-tagged Mso1p localizes to the sites of exocytosis and at the site of prospore membrane formation. In vivo and in vitro experiments identified a short amino-terminal sequence in Mso1p that mediates its interaction with Sec1p and is needed for vesicle fusion. A point mutation, T47A, within the Sec1p-binding domain abolishes Mso1p functionality in vivo, and mso1T47A mutant cells display specific genetic interactions with sec1 mutants. Mso1p coimmunoprecipitates with Sec1p, Sso1/2p, Snc1/2p, Sec9p, and the exocyst complex subunit Sec15p. In sec4-8 and SEC4I133 mutant cells, association of Mso1p with Sso1/2p, Snc1/2p, and Sec9p is affected, whereas interaction with Sec1p persists. Furthermore, in SEC4I133 cells the dominant negative Sec4I133p coimmunoprecipitates with Mso1p-Sec1p complex. Finally, we identify Mso1p as a homologue of the PTB binding domain of the mammalian Sec1p binding Mint proteins. These results position Mso1p in the interface of the exocyst complex, Sec4p, and the SNARE machinery, and reveal a novel layer of molecular conservation in the exocytosis machinery.
Figures





Similar articles
-
A conserved regulatory mode in exocytic membrane fusion revealed by Mso1p membrane interactions.Mol Biol Cell. 2013 Feb;24(3):331-41. doi: 10.1091/mbc.E12-05-0415. Epub 2012 Nov 28. Mol Biol Cell. 2013. PMID: 23197474 Free PMC article.
-
Sec1p and Mso1p C-terminal tails cooperate with the SNAREs and Sec4p in polarized exocytosis.Mol Biol Cell. 2011 Jan 15;22(2):230-44. doi: 10.1091/mbc.E10-07-0592. Epub 2010 Nov 30. Mol Biol Cell. 2011. PMID: 21119007 Free PMC article.
-
Mso1p regulates membrane fusion through interactions with the putative N-peptide-binding area in Sec1p domain 1.Mol Biol Cell. 2010 Apr 15;21(8):1362-74. doi: 10.1091/mbc.e09-07-0546. Epub 2010 Feb 24. Mol Biol Cell. 2010. PMID: 20181830 Free PMC article.
-
The role of Sec1p-related proteins in vesicle trafficking in the nerve terminal.J Neurosci Res. 1996 Jul 15;45(2):89-95. doi: 10.1002/(SICI)1097-4547(19960715)45:2<89::AID-JNR1>3.0.CO;2-B. J Neurosci Res. 1996. PMID: 8843026 Review.
-
SNARE Protein Snc1 Is Essential for Vesicle Trafficking, Membrane Fusion and Protein Secretion in Fungi.Cells. 2023 Jun 5;12(11):1547. doi: 10.3390/cells12111547. Cells. 2023. PMID: 37296667 Free PMC article. Review.
Cited by
-
A conserved regulatory mode in exocytic membrane fusion revealed by Mso1p membrane interactions.Mol Biol Cell. 2013 Feb;24(3):331-41. doi: 10.1091/mbc.E12-05-0415. Epub 2012 Nov 28. Mol Biol Cell. 2013. PMID: 23197474 Free PMC article.
-
Pandemism of swine flu and its prospective drug therapy.Eur J Clin Microbiol Infect Dis. 2012 Dec;31(12):3265-79. doi: 10.1007/s10096-012-1716-5. Epub 2012 Aug 16. Eur J Clin Microbiol Infect Dis. 2012. PMID: 22895890 Review.
-
DevS, a heme-containing two-component oxygen sensor of Mycobacterium tuberculosis.Biochemistry. 2007 Apr 10;46(14):4250-60. doi: 10.1021/bi602422p. Epub 2007 Mar 20. Biochemistry. 2007. PMID: 17371046 Free PMC article.
-
Yarrowia lipolytica vesicle-mediated protein transport pathways.BMC Evol Biol. 2007 Nov 12;7:219. doi: 10.1186/1471-2148-7-219. BMC Evol Biol. 2007. PMID: 17997821 Free PMC article.
-
The Use of Saccharomyces cerevisiae Supplemented with Intracellular Magnesium Ions by Means of Pulsed Electric Field (PEF) in the Process of Bread Production.Foods. 2022 Nov 3;11(21):3496. doi: 10.3390/foods11213496. Foods. 2022. PMID: 36360110 Free PMC article.
References
-
- Biederer, T., and Sudhof, T. C. (2000). Mints as adaptors. Direct binding to neurexins and recruitment of munc18. J. Biol. Chem. 275, 39803–39806. - PubMed
-
- Borg, J. P., Straight, S. W., Kaech, S. M., de Taddeo-Borg, M., Kroon, D. E., Karnak, D., Turner, R. S., Kim, S. K., and Margolis, B. (1998). Identification of an evolutionarily conserved heterotrimeric protein complex involved in protein targeting. J. Biol. Chem. 273, 31633–31636. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

