Modulator effects of interleukin-1beta and tumor necrosis factor-alpha on AMPA-induced excitotoxicity in mouse organotypic hippocampal slice cultures
- PMID: 16033883
- PMCID: PMC6725352
- DOI: 10.1523/JNEUROSCI.1510-05.2005
Modulator effects of interleukin-1beta and tumor necrosis factor-alpha on AMPA-induced excitotoxicity in mouse organotypic hippocampal slice cultures
Abstract
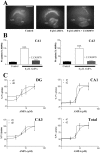
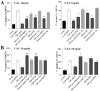
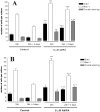
The inflammatory cytokines interleukin-1beta and tumor necrosis factor-alpha (TNF-alpha) have been identified as mediators of several forms of neurodegeneration in the brain. However, they can produce either deleterious or beneficial effects on neuronal function. We investigated the effects of these cytokines on neuronal death caused by exposure of mouse organotypic hippocampal slice cultures to toxic concentrations of AMPA. Either potentiation of excitotoxicity or neuroprotection was observed, depending on the concentration of the cytokines and the timing of exposure. A relatively high concentration of mouse recombinant TNF-alpha (10 ng/ml) enhanced excitotoxicity when the cultures were simultaneously exposed to AMPA and to this cytokine. Decreasing the concentration of TNF-alpha to 1 ng/ml resulted in neuroprotection against AMPA-induced neuronal death independently on the application protocol. By using TNF-alpha receptor (TNFR) knock-out mice, we demonstrated that the potentiation of AMPA-induced toxicity by TNF-alpha involves TNF receptor-1, whereas the neuroprotective effect is mediated by TNF receptor-2. AMPA exposure was associated with activation and proliferation of microglia as assessed by macrophage antigen-1 and bromodeoxyuridine immunohistochemistry, suggesting a functional recruitment of cytokine-producing cells at sites of neurodegeneration. Together, these findings are relevant for understanding the role of proinflammatory cytokines and microglia activation in acute and chronic excitotoxic conditions.
Figures





References
-
- Allan SM, Rothwell NJ (2001) Cytokines and acute neurodegeneration. Nat Rev Neurosci 2: 734-744. - PubMed
-
- Balosso S, Ravizza T, Perego C, Peschon J, Campbell IL, De Simoni MG, Vezzani A (2005) Tumor necrosis factor-α inhibits seizures in mice via p75 receptors. Ann Neurol 57: 804-812. - PubMed
-
- Barger SW, Horster D, Furukawa K, Goodman Y, Krieglstein J, Mattson MP (1995) Tumor necrosis factors alpha and beta protect neurons against amyloid beta-peptide toxicity: evidence for involvement of a kappaB-binding factor and attenuation of peroxide and Ca2+ accumulation. Proc Natl Acad Sci USA 92: 9328-9332. - PMC - PubMed
-
- Beattie EC, Stellwagen D, Morishita W, Bresnahan JC, Ha BK, Von Zastrow M, Beattie MS, Malenka RC (2002) Control of synaptic strength by glial TNFalpha. Science 295: 2282-2285. - PubMed
-
- Beattie MS (2004) Inflammation and apoptosis: linked therapeutic targets in spinal cord injury. Trends Mol Med 10: 580-583. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
