FasL (CD95L/APO-1L) resistance of neurons mediated by phosphatidylinositol 3-kinase-Akt/protein kinase B-dependent expression of lifeguard/neuronal membrane protein 35
- PMID: 16033886
- PMCID: PMC6725360
- DOI: 10.1523/JNEUROSCI.1700-05.2005
FasL (CD95L/APO-1L) resistance of neurons mediated by phosphatidylinositol 3-kinase-Akt/protein kinase B-dependent expression of lifeguard/neuronal membrane protein 35
Abstract
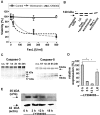
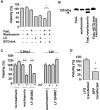
The contribution of Fas (CD95/APO-1) to cell death mechanisms of differentiated neurons is controversially discussed. Rat cerebellar granule neurons (CGNs) express high levels of Fas in vitro but are resistant to FasL (CD95L/APO-1L/CD178)-induced apoptosis. We here show that this resistance was mediated by a phosphatidylinositol 3-kinase (PI 3-kinase)-Akt/protein kinase B (PKB)-dependent expression of lifeguard (LFG)/neuronal membrane protein 35. Reduction of endogenous LFG expression by antisense oligonucleotides or small interfering RNA lead to increased sensitivity of CGNs to FasL-induced cell death and caspase-8 cleavage. The inhibition of PI 3-kinase activity sensitized CGNs to FasL-induced caspase-8 and caspase-3 processing and caspase-dependent fodrin cleavage. Pharmacological inhibition of PI 3-kinase, overexpression of the inhibitory protein IkappaB, or cotransfection of an LFG reporter plasmid with dominant-negative Akt/PKB inhibited LFG reporter activity, whereas overexpression of constitutively active Akt/PKB increased LFG reporter activity. Overexpression of LFG in CGNs interfered with the sensitization to FasL by PI 3-kinase inhibitors. In contrast to CGNs, 12 glioma cell lines, which are sensitive to FasL, did not express LFG. Gene transfer of LFG into these FasL-susceptible glioma cells protected against FasL-induced apoptosis. These results demonstrate that LFG mediated the FasL resistance of CGNs and that, under certain circumstances, e.g., inhibition of the PI 3-kinase-Akt/PKB pathway, CGNs were sensitized to FasL.
Figures




References
-
- Bechmann I, Mor G, Nilsen J, Eliza M, Nitsch R, Naftolin F (1999) FasL (CD95L, Apo1L) is expressed in the normal rat and human brain: evidence for the existence of an immunological brain barrier. Glia 27: 62-74. - PubMed
-
- Bellacosa A, Testa JR, Staal SP, Tsichlis PN (1991) A retroviral oncogene, akt, encoding a serine-threonine kinase containing an SH2-like region. Science 254: 274-277. - PubMed
-
- Brummelkamp TR, Bernards R, Agami R (2002) A system for stable expression of short interfering RNAs in mammalian cells. Science 296: 550-553. - PubMed
-
- Brunet A, Bonni A, Zigmond MJ, Lin MZ, Juo P, Hu LS, Anderson MJ, Arden KC, Blenis J, Greenberg ME (1999) Akt promotes cell survival by phosphorylating and inhibiting a Forkhead transcription factor. Cell 96: 857-868. - PubMed
-
- Burgering BMT, Coffer PJ (1995) Protein kinase B (c-Akt) in phosphatidylinositol-3-OH kinase signal transduction. Nature 376: 599-602. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous
