Neonatal and adult neurogenesis provide two distinct populations of newborn neurons to the mouse olfactory bulb
- PMID: 16033891
- PMCID: PMC6725349
- DOI: 10.1523/JNEUROSCI.1114-05.2005
Neonatal and adult neurogenesis provide two distinct populations of newborn neurons to the mouse olfactory bulb
Abstract
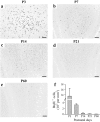
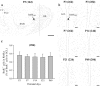
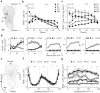
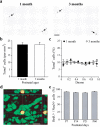
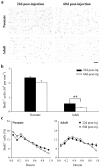
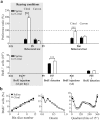
In mammals, the olfactory bulb (OB) constitutes one of two regions of the postnatal brain with continuous neurogenesis throughout life. Despite intense explorations of neuronal replacement in the adult OB, little is known about the mechanisms that operate at earlier postnatal stages. This question is particularly pertinent, because the majority of local interneurons are born in the neonate, when olfaction controls vital functions. Here, we analyzed the recruitment of newborn cells to the granule cell (GC) layer (GCL) and found that the postnatal mouse OB is supplied with two spatiotemporally distinct populations of newborn interneurons. Early born [postnatal day 3 (P3) to P7] GCs constitute a threefold larger population compared with those generated later (P14-P60), and some of them are produced locally within the OB itself. Newborn interneurons generated at P3-P7 were predominantly targeted to the external edge of the GCL, whereas newly generated cells were positioned deeper in older mice. Additionally, although approximately 50% of adult newborn cells were eliminated within a few weeks of reaching the OB, almost the entire population of early born GCs survived until adulthood. Importantly, early olfactory experience specifically modifies the number of newborn GCs in neonates but leaves unaltered the amount of neurons generated during adulthood. Together, these results demonstrate that early postnatal neurogenesis endows the neonate bulbar circuit with newborn GCs that differ morphologically and functionally from those produced in the adult.
Figures

References
-
- Altman J (1969) Autoradiographic and histological studies of postnatal neurogenesis. IV. Cell proliferation and migration in the anterior forebrain, with special reference to persisting neurogenesis in the olfactory bulb. J Comp Neurol 137: 433-457. - PubMed
-
- Altman J, Das GD (1966) Autoradiographic and histological studies of postnatal neurogenesis. I. A longitudinal investigation of the kinetics, migration and transformation of cells incorporating tritiated thymidine in neonate rats, with special reference to postnatal neurogenesis in some brain regions. J Comp Neurol 126: 337-389. - PubMed
-
- Bayer SA (1983) 3H-Thymidine-radiographic studies of neurogenesis in the rat olfactory bulb. Exp Brain Res 50: 329-340. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous
