Synaptically driven endocannabinoid release requires Ca2+-assisted metabotropic glutamate receptor subtype 1 to phospholipase Cbeta4 signaling cascade in the cerebellum
- PMID: 16033892
- PMCID: PMC6725357
- DOI: 10.1523/JNEUROSCI.0945-05.2005
Synaptically driven endocannabinoid release requires Ca2+-assisted metabotropic glutamate receptor subtype 1 to phospholipase Cbeta4 signaling cascade in the cerebellum
Abstract
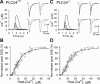
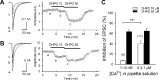
Endocannabinoids mediate retrograde signaling and modulate synaptic transmission in various regions of the CNS. Depolarization-induced elevation of intracellular Ca2+ concentration causes endocannabinoid-mediated suppression of excitatory/inhibitory synaptic transmission. Activation of G(q/11)-coupled receptors including group I metabotropic glutamate receptors (mGluRs) also causes endocannabinoid-mediated suppression of synaptic transmission. However, precise mechanisms of endocannabinoid production initiated by physiologically relevant synaptic activity remain to be determined. To address this problem, we made whole-cell recordings from Purkinje cells (PCs) in mouse cerebellar slices and examined their excitatory synapses arising from climbing fibers (CFs) and parallel fibers (PFs). We first characterized three distinct modes to induce endocannabinoid release by analyzing CF to PC synapses. The first mode is strong activation of mGluR subtype 1 (mGluR1)-phospholipase C (PLC) beta4 cascade without detectable Ca2+ elevation. The second mode is Ca2+ elevation to a micromolar range without activation of the mGluR1-PLCbeta4 cascade. The third mode is the Ca2+-assisted mGluR1-PLCbeta4 cascade that requires weak mGluR1 activation and Ca2+ elevation to a submicromolar range. By analyzing PF to PC synapses, we show that the third mode is essential for effective endocannabinoid release from PCs by excitatory synaptic activity. Furthermore, our biochemical analysis demonstrates that combined weak mGluR1 activation and mild depolarization in PCs effectively produces 2-arachidonoylglycerol (2-AG), a candidate of endocannabinoid, whereas either stimulus alone did not produce detectable 2-AG. Our results strongly suggest that under physiological conditions, excitatory synaptic inputs to PCs activate the Ca2+-assisted mGluR1-PLCbeta4 cascade, and thereby produce 2-AG, which retrogradely modulates synaptic transmission to PCs.
Figures




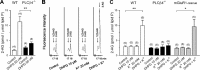
References
-
- Aiba A, Kano M, Chen C, Stanton ME, Fox GD, Herrup K, Zwingman TA, Tonegawa S (1994) Deficient cerebellar long-term depression and impaired motor learning in mGluR1 mutant mice. Cell 79: 377-388. - PubMed
-
- Alger BE (2002) Retrograde signaling in the regulation of synaptic transmission: focus on endocannabinoids. Prog Neurobiol 68: 247-286. - PubMed
-
- Bisogno T, Howell F, Williams G, Minassi A, Cascio MG, Ligresti A, Matias I, Schiano-Moriello A, Paul P, Williams EJ, Gangadharan U, Hobbs C, Di Marzo V, Doherty P (2003) Cloning of the first sn1-DAG lipases points to the spatial and temporal regulation of endocannabinoid signaling in the brain. J Cell Biol 163: 463-468. - PMC - PubMed
-
- Brenowitz SD, Regehr WG (2005) Associative short-term synaptic plasticity mediated by endocannabinoids. Neuron 45: 419-431. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous
