Endocannabinoid signaling in rat somatosensory cortex: laminar differences and involvement of specific interneuron types
- PMID: 16033894
- PMCID: PMC6725346
- DOI: 10.1523/JNEUROSCI.0442-05.2005
Endocannabinoid signaling in rat somatosensory cortex: laminar differences and involvement of specific interneuron types
Abstract
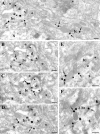
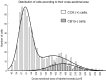
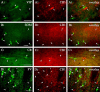
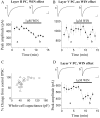
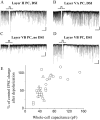
Endocannabinoid-mediated retrograde signaling exerts powerful control over synaptic transmission in many brain areas. However, in the neocortex, the precise laminar, cellular, and subcellular localization of the type 1 cannabinoid receptor (CB1) as well as its function has been elusive. Here we combined multiple immunolabeling with whole-cell recordings to investigate the morpho-functional characteristics of cannabinoid signaling in rat somatosensory cortex. Immunostaining for CB1 revealed axonal and somatic labeling with striking layer specificity: a high density of CB1-positive fibers was seen in layers II-III, in layer VI, and in upper layer V, whereas other layers had sparse (layer IV) or hardly any (layer I) staining. Membrane staining for CB1 was only found in axon terminals, all of which contained GABA and formed symmetric synapses. Double immunostaining also revealed that CB1-positive cells formed two neurochemically distinct subpopulations: two-thirds were cholecystokinin positive and one-third expressed calbindin, each subserving specific inhibitory functions in cortical networks. In addition, cannabinoid sensitivity of GABAergic input showed striking layer specificity, as revealed by both electrophysiological and anatomical experiments. We found a unique population of large pyramidal neurons in layer VB that received much less perisomatic innervation from CB1-expressing GABAergic axon terminals and, accordingly, showed no depolarization-induced suppression of inhibition, unlike pyramidal cells in layer II, and a population of small pyramidal cells in layer V. This suggests that inhibitory control of pyramidal cells involved in intracortical or corticostriatal processing is fine-tuned by activity-dependent endocannabinoid signaling, whereas inhibition of pyramidal cells relaying cortical information to lower subcortical effector centers often lacks this plasticity.
Figures






References
-
- Alger BE (2002) Retrograde signaling in the regulation of synaptic transmission: focus on endocannabinoids. Prog Neurobiol 68: 247-286. - PubMed
-
- Bacci A, Huguenard JR, Prince DA (2004) Long-lasting self-inhibition of neocortical interneurons mediated by endocannabinoids. Nature 431: 312-316. - PubMed
-
- Baimbridge KG, Miller JJ, Parkes CO (1982) Calcium-binding protein distribution in the rat brain. Brain Res 239: 519-525. - PubMed
-
- Berod A, Hartman BK, Pujol JF (1981) Importance of fixation in immunohistochemistry: use of formaldehyde solutions at variable pH for the localization of tyrosine hydroxylase. J Histochem Cytochem 29: 844-850. - PubMed
-
- Carlson G, Wang Y, Alger BE (2002) Endocannabinoids facilitate the induction of LTP in the hippocampus. Nat Neurosci 5: 723-724. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
