Evidence for an instructive role of retinal activity in retinotopic map refinement in the superior colliculus of the mouse
- PMID: 16033903
- PMCID: PMC6725341
- DOI: 10.1523/JNEUROSCI.1470-05.2005
Evidence for an instructive role of retinal activity in retinotopic map refinement in the superior colliculus of the mouse
Abstract
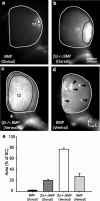
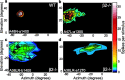
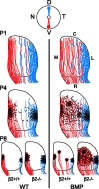
Although it is widely accepted that molecular mechanisms play an important role in the initial establishment of retinotopic maps, it has also long been argued that activity-dependent factors act in concert with molecular mechanisms to refine topographic maps. Evidence of a role for retinal activity in retinotopic map refinement in mammals is limited, and nothing is known about the effect of spontaneous retinal activity on the development of receptive fields in the superior colliculus. Using anatomical and physiological methods with two genetically manipulated mouse models and pharmacological interventions in wild-type mice, we show that spontaneous retinal waves instruct retinotopic map refinement in the superior colliculus of the mouse. Activity-dependent mechanisms may play a preferential role in the mapping of the nasal-temporal axis of the retina onto the colliculus, because refinement is particularly impaired along this axis in mutants without retinal waves. Interfering with both axon guidance cues and activity-dependent cues in the same animal has a dramatic cumulative effect. These experiments demonstrate how axon guidance cues and activity-dependent factors combine to instruct retinotopic map development.
Figures




References
-
- Baldi P, Heiligenberg W (1988) How sensory maps could enhance resolution through ordered arrangements of broadly tuned receivers. Biol Cybern 59: 313-318. - PubMed
-
- Bansal A, Singer JH, Hwang BJ, Xu W, Beaudet A, Feller MB (2000) Mice lacking specific nicotinic acetylcholine receptor subunits exhibit dramatically altered spontaneous activity patterns and reveal a limited role for retinal waves in forming ON and OFF circuits in the inner retina. J Neurosci 20: 7672-7681. - PMC - PubMed
-
- Carrasco MM, Razak KA, Pallas SL (2003) Effects of dark-rearing on retinocollicular map refinement. Soc Neurosci Abstr 29: 37.23.
-
- Chan SO, Guillery RW (1994) Changes in fiber order in the optic nerve and tract of rat embryos. J Comp Neurol 344: 20-32. - PubMed
-
- Chen C, Regehr WG (2000) Developmental remodeling of the retinogeniculate synapse. Neuron 28: 955-966. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
