A shared epitope of the interphotoreceptor retinoid-binding protein recognized by the CD4+ and CD8+ autoreactive T cells
- PMID: 16034128
- PMCID: PMC4219322
- DOI: 10.4049/jimmunol.175.3.1851
A shared epitope of the interphotoreceptor retinoid-binding protein recognized by the CD4+ and CD8+ autoreactive T cells
Abstract
We previously demonstrated that cultures of rat uveitogenic T cells rapidly become dominated by CD4+ cells, but activation of CD8+ autoreactive T cells also occurred during the in vitro culture of in vivo-primed T cells. In the present study, we show that the commonly used uveitogenic peptide, interphotoreceptor retinoid-binding protein (IRBP) 1-20, generated both CD4+ and CD8+ autoreactive T cells in the C57BL/6 (B6) mouse and that this 20-mer contains at least two distinct antigenic epitopes. To determine whether the CD8 response was Ag-specific and whether CD4+ and CD8+ IRBP1-20-specific T cells recognize distinct antigenic epitopes, we prepared highly purified CD4+ and CD8+ T cells from IRBP1-20-primed mice and tested their proliferative response to a large panel of truncated peptides derived from IRBP1-20. The results showed that both CD4+ and CD8+ T cells recognized the same spectrum of peptides. In addition, peptides P10-18 were found to bind effectively to CD8+ IRBP1-20-specific T cells when complexed with recombinant H-2K(b) and also stimulate the proliferation and cytokine production of CD4+ IRBP1-20-specific T cells. Our results document for the first time that CD8+ and CD4+ autoreactive T cells display characteristic epitope recognition and they both recognize the same core epitope.
Figures

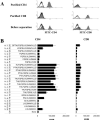

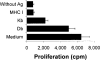


Similar articles
-
Sequence 168 to 177 of interphotoreceptor retinoid-binding protein (IRBP) is an antigenic epitope for autoreactive CD8 T cells in the B10RIII mouse.J Neuroimmunol. 2008 Jan;193(1-2):68-76. doi: 10.1016/j.jneuroim.2007.10.016. Epub 2007 Dec 11. J Neuroimmunol. 2008. PMID: 18063114 Free PMC article.
-
Repertoire analysis and new pathogenic epitopes of IRBP in C57BL/6 (H-2b) and B10.RIII (H-2r) mice.Invest Ophthalmol Vis Sci. 2008 May;49(5):1946-56. doi: 10.1167/iovs.07-0868. Invest Ophthalmol Vis Sci. 2008. PMID: 18436827 Free PMC article.
-
In vitro activation of CD8 interphotoreceptor retinoid-binding protein-specific T cells requires not only antigenic stimulation but also exogenous growth factors.J Immunol. 2006 Apr 15;176(8):5006-14. doi: 10.4049/jimmunol.176.8.5006. J Immunol. 2006. PMID: 16585597
-
Characterization of IL-17+ interphotoreceptor retinoid-binding protein-specific T cells in experimental autoimmune uveitis.Invest Ophthalmol Vis Sci. 2007 Sep;48(9):4153-61. doi: 10.1167/iovs.07-0251. Invest Ophthalmol Vis Sci. 2007. PMID: 17724201 Free PMC article.
-
Major role of gamma delta T cells in the generation of IL-17+ uveitogenic T cells.J Immunol. 2009 Jul 1;183(1):560-7. doi: 10.4049/jimmunol.0900241. J Immunol. 2009. PMID: 19542467 Free PMC article.
Cited by
-
Sequence 168 to 177 of interphotoreceptor retinoid-binding protein (IRBP) is an antigenic epitope for autoreactive CD8 T cells in the B10RIII mouse.J Neuroimmunol. 2008 Jan;193(1-2):68-76. doi: 10.1016/j.jneuroim.2007.10.016. Epub 2007 Dec 11. J Neuroimmunol. 2008. PMID: 18063114 Free PMC article.
-
Amelioration of experimental autoimmune uveoretinitis by aldose reductase inhibition in Lewis rats.Invest Ophthalmol Vis Sci. 2011 Oct 11;52(11):8033-41. doi: 10.1167/iovs.11-7485. Invest Ophthalmol Vis Sci. 2011. PMID: 21900376 Free PMC article.
-
A novel pathogenic RBP-3 peptide reveals epitope spreading in persistent experimental autoimmune uveoretinitis.Immunology. 2015 Oct;146(2):301-11. doi: 10.1111/imm.12503. Epub 2015 Aug 11. Immunology. 2015. PMID: 26152845 Free PMC article.
-
Connection between γδ T-cell- and Adenosine- Mediated Immune Regulation in the Pathogenesis of Experimental Autoimmune Uveitis.Crit Rev Immunol. 2018;38(3):233-243. doi: 10.1615/CritRevImmunol.2018026150. Crit Rev Immunol. 2018. PMID: 30004859 Free PMC article. Review.
-
Timing Effect of Adenosine-Directed Immunomodulation on Mouse Experimental Autoimmune Uveitis.J Immunol. 2021 Jul 1;207(1):153-161. doi: 10.4049/jimmunol.2100182. Epub 2021 Jun 14. J Immunol. 2021. PMID: 34127521 Free PMC article.
References
-
- Fox GM, Kuwabara T, Wiggert B, Redmond TM, Hess HH, Chader GJ, Gery I. Experimental autoimmune uveoretinitis (EAU) induced by retinal interphotoreceptor retinoid-binding protein (IRBP): differences between EAU induced by IRBP and by S-antigen. Clin. Immunol. Immunopathol. 1987;43:256–264. - PubMed
-
- Silver PB, Rizzo LV, Chan CC, Donoso LA, Wiggert B, Caspi RR. Identification of a major pathogenic epitope in the human IRBP molecule recognized by mice of the H-2r haplotype. Invest. Ophthalmol. Vis. Sci. 1995;36:946–954. - PubMed
-
- Avichezer D, Silver PB, Chan CC, Wiggert B, Caspi RR. Identification of a new epitope of human IRBP that induces autoimmune uveoretinitis in mice of the H-2b haplotype. Invest. Ophthalmol. Vis. Sci. 2000;41:127–131. - PubMed
-
- Rizzo LV, Silver P, Wiggert B, Hakim F, Gazzinelli RT, Chan CC, Caspi RR. Establishment and characterization of a murine CD4+ T cell line and clone that induce experimental autoimmune uveoretinitis in B10.A mice. J. Immunol. 1996;156:1654–1660. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

