Sertindole for schizophrenia
- PMID: 16034864
- PMCID: PMC7025766
- DOI: 10.1002/14651858.CD001715.pub2
Sertindole for schizophrenia
Abstract
Background: Sertindole is an atypical antipsychotic, which is thought to give a lower incidence of extrapyramidal side effects at clinically effective doses than typical antipsychotic drugs. In December 1998, Lundbeck Ltd., the manufacturers of sertindole, voluntarily suspended the availability of the drug due to concerns about cardiac arrhythmia and sudden cardiac death associated with its use. However, based on the advice of an appointed expert group, the Committee for Proprietary Medicinal Products (CPMP) lifted the suspension of sertindole in October 2001, a decision that was ratified by the European Commission on the 26th of June 2002. Lundbeck have committed to the CPMP to carry out two post-marketing surveillance (PMS) studies (which were initiated in July 2002) to provide additional epidemiological data under conditions of normal drug usage. Initial marketing of the product will be restricted and Lundbeck is currently in discussions with the US health authorities (FDA) to investigate whether, and if so when, it would be possible to launch Serdolect in the US market.
Objectives: To determine the effects of sertindole compared with placebo, typical and other atypical antipsychotic drugs for schizophrenia and related psychoses.
Search strategy: Our Initial searches included electronic searches of Biological Abstracts (1980-1999), The Cochrane Library (Issue 1, 1999), The Cochrane Schizophrenia Group's Register (August 2000), EMBASE (1980-1999), LILACS (1982-1996), MEDLINE (1966-1999), PSYNDEX (1977-1995) and PsycLIT (1974-1999). In addition, we searched pharmaceutical databases on the Dialog Corporation Datastar and Dialog services. We searched references of all identified studies for further trials. We contacted the manufacturer of sertindole and authors of trials. We updated the literature search by searching the Cochrane Schizophrenia Group's Trials Register in April 2003.
Selection criteria: All randomised controlled trials that compared sertindole to placebo or other antipsychotic (atypical or typical) drug treatments for patients with schizophrenia or related psychosis .
Data collection and analysis: We independently inspected citations and, where possible abstracts; ordered papers for re-inspection and quality assessment and independently extracted data. For homogeneous dichotomous data, we calculated the risk ratio (RR), 95% confidence interval (CI) and, where appropriate, the number needed to treat (NNT) or number needed to harm (NNH) on an intention-to-treat basis. For continuous data, we calculated weighted mean differences (WMD). We inspected all data for heterogeneity.
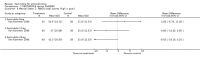
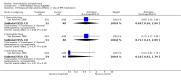
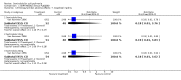
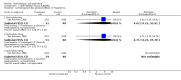
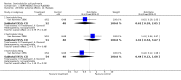
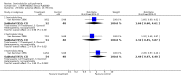
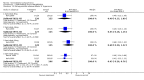
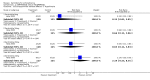
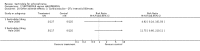
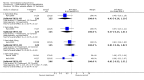
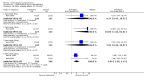
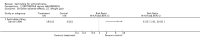
Main results: Currently the review includes three studies with a total of 1,104 participants. One was a medium term (eight weeks) placebo controlled study that examined three different doses of sertindole (8, 12 and 20mg/day). The remaining two studies compared the use of sertindole with haloperidol (10mg/day). One was a short term study (six weeks) that looked at four different doses of sertindole (8, 16, 20, 24mg/day) and the other was a long term study (one year) that evaluated the use of sertindole 24mg/day in participants attending outpatients. We excluded two large important studies because they did not report any usable data. (Both had greater than 50% loss to follow-up and data on 'leaving the study early' was inadequately reported). SERTINDOLE VERSUS PLACEBO: Sertindole at 20mg/day was found to be more effective than placebo in terms of BPRS total scores (1 study, n=78, MD 6.2, CI -11.8 to -0.6) and CGI total end point scores (1 study, n=78, MD -0.9, CI -1.6 to -0.2). A marginally statistically significantly greater number of participants that were treated with 20 mg of sertindole were reported to have been 'very much improved' as compared to those taking placebo (1 study, n=102, RR 7.6, CI 1.0 to 57.9, NNT 7.9, CI 4.3 to 41.1). There was no statistically significant difference between sertindole at 8 or 12 mg/day and placebo for these three outcome measures. There were no statistically significant differences between sertindole (8, 12 or 20 mg) and placebo for the incidence of extrapyramidal symptoms, extrapyramidal related events or use of medication to avoid extrapyramidal symptoms. There were no statistically significant differences found between sertindole and placebo for the movement disorders akathisia, cogwheel rigidity, hypertonia and tremor or somnolence. At eight weeks a statistically significant difference between placebo and all sertindole groups (8, 12 and 20 mg) for mean change from baseline in the QT and QTc intervals were observed (p values and SD were not reported). There was a statistically significant greater mean weight gain among participants taking sertindole (20 mg, mean weight gain of 3.3 kg) as compared to placebo (mean weight gain of 0.8 kg; p<0.05). SERTINDOLE VERSUS HALOPERIDOL: At one year, a greater number of participants who were treated with haloperidol as compared to sertindole (24mg/day) were leaving the study early due to any reason (1 study, n=282, RR 0.6, CI 0.4 to 1.0, NNH 8.8, CI 4.7 to 74.0) or non-compliance (1 study, n=282, RR 0.2, CI 0.0 to 0.7, NNH 12.8, CI 7.7 to 37.8). However, at six weeks, there was no statistically significant difference between sertindole (at 8, 16, 20, or 24mg) and haloperidol for this latter outcome. The incidence of EPS was higher among those treated with haloperidol than sertindole at 8, 16, 20 or 24mg/day (8mg: 1 study, n=245, RR 0.1, CI 0.0 to 0.7, NNH 11.4, CI 7.1 to 29.8; 16mg: 1 study, n=252, RR 0.3, CI 0.1 to 1.0, NNH 15.5, CI 8.0 to 217.9; and 20mg: 1 study, n=253, RR 0.2, CI 0.1 to 0.8, NNH 13.7, CI 7.7 to 68.3; 24mg: 2 studies, n=524, RR 0.6, CI 0.4 to 0.8, NNH 8.7, CI 5.4 to 23.0). More participants treated with haloperidol experienced akathisia, tremor and hypertonia than those treated with sertindole (Akathisia - 8mg: 1 study, n=245, RR 0.2, CI 0.1 to 0.5, NNH 6.0, CI 4.1 to 11.2; 16mg: 1 study, n=252, RR 0.1, CI 0.0 to 0.3, NNH 5.4, CI 3.9 9.0; 20mg: 1 study, n=253, RR 0.3, CI 0.2 to 0.7, NNH 7.3, CI 4.6 to 17.9; 24mg: 2 studies, n=524, RR 0.5, CI 0.3 to 0.7, NNH 8.6, CI 5.6 to 18.3. Tremor - 8mg: 1 study, n=245, RR 0.3, CI 0.1 to 0.7, NNH 8.5, CI 5.2 to 24.0; 16mg: 1 study, n=252, RR 0.2, CI 0.1 to 0.5, NNH 7.3, 4.8 to 15.6; 20mg: 1 study, n=253, RR 0.2, CI 0.1 to 0.6, NNH 7.8, CI 4.9 to 18.1; 24mg: 2 studies, n=524, RR 0.4, CI 0.2 to 0.6, NNH 8.2, CI 5.6 to 15.3. For Hypertonic - 24mg: 2 studies, n=524, RR 0.5, CI 0.3 to 0.8, NNH 12.4, CI 7.5 to 35.0; for sertindole 8, 16 and 20mg there was no statistically significant differences between the treatment groups). One study reported that at six weeks, there was a statistically significant greater increase from baseline to final value in mean QTc interval in the sertindole 16, 20 and 24mg groups (20, 26, and 24msec, respectively) than in the haloperidol group (0msec; p value was not reported), but no SD or any other measure of variance for the effect sizes were reported. For one long term study only one participant from the sertindole group (24mg) had a QT interval that exceeded 500msec (1 study, n=282, RR 3.0 CI 0.1 to 73.0), but 11participants treated with Sertindole had QTc intervals of at least 500msec, compared to none in the haloperidol treated group (1 study, n=282, RR 23.0, CI 1.4 to 386.6, NNH 12.8, CI 8.2 to 29.6). At six weeks, fewer participants treated with sertindole at 8mg or 24mg were affected by somnolence than those treated with haloperidol (sertindole 8mg: 1 study, n=245, RR 0.1, CI 0.0 to 0.7, NNH 11.4, CI 7.1 to 29.8; 24mg: 2 studies, n=524, RR 0.6, CI 0.4 to 1.0, NNH 14.8, CI 7.7 to 205.2). The incidence of rhinitis was found to be statistically significantly higher among those taking sertindole at 16 or 24mg as compared to haloperidol (16mg: 1 study, n=252, RR 10.8, CI 1.4 to 82.6, NNH 12.7, CI 7.7 to 36.7; 24mg: 2 studies, n= 524, RR 2.1, CI 1.4 to 3.1, NNH 8.7, CI 5.6 to 18.6). At one year, 33 participants treated with sertindole (24mg) had experienced the sexual adverse event of decreased ejaculatory volume, compared with six participants treated with haloperidol. However the number of included male participants was not reported and therefore the RR could not be calculated. At one year, more participants taking sertindole (24mg/day) had put on weight compared to those taking haloperidol (1 study, n=282, RR 6.3, CI 1.9 to 20.9, NNH 8.8, CI 5.7 to 19.1). At six weeks, all of the sertindole groups showed an increase in body weight from baseline to final evaluation ranging from 1.3kg to 1.9kg, all of which represented a statistically significantly different weight change than that recorded for the haloperidol treatment group (-0.1Kg). However, the actual weight gain for each sertindole dosage group was not reported and no SD or any other measure of variance was given.
Authors' conclusions: Sertindole at a dose of 20mg/day was found to be more antipsychotic than placebo. When used at 8, 12 or 20mg/day it appears to be as acceptable as placebo (in terms of various adverse events including movement disorders and somnolence), but seems to be associated with more cardiac problems (8, 12 or 20mg/day) and an increase in weight gain (20mg/day) than placebo. Sertindole at a dose of 24mg/day was better tolerated than haloperidol (in terms of participants leaving the study early). It was also found to be was associated with fewer movement disorders (at 8, 16, 20 or 24mg/day) and sedation (8 or 24mg/day) than haloperidol. However, it was shown to cause more cardiac anomalies (16, 20 or 24mg/day), weight gain (all doses combined), rhinitis (16 or 24mg/day), and problems with sexual functioning (24mg/day) than haloperidol. One short term study reported that sertindole 16mg/day was the most optimal dose.
Conflict of interest statement
None known
Figures






















































Update of
-
Sertindole for schizophrenia.Cochrane Database Syst Rev. 2000;(2):CD001715. doi: 10.1002/14651858.CD001715. Cochrane Database Syst Rev. 2000. Update in: Cochrane Database Syst Rev. 2005 Jul 20;(3):CD001715. doi: 10.1002/14651858.CD001715.pub2. PMID: 10796657 Updated.
Similar articles
-
Sertindole for schizophrenia.Cochrane Database Syst Rev. 2000;(2):CD001715. doi: 10.1002/14651858.CD001715. Cochrane Database Syst Rev. 2000. Update in: Cochrane Database Syst Rev. 2005 Jul 20;(3):CD001715. doi: 10.1002/14651858.CD001715.pub2. PMID: 10796657 Updated.
-
Risperidone versus other atypical antipsychotics for schizophrenia.Cochrane Database Syst Rev. 2011 Jan 19;2011(1):CD006626. doi: 10.1002/14651858.CD006626.pub2. Cochrane Database Syst Rev. 2011. PMID: 21249678 Free PMC article.
-
Amisulpride for schizophrenia.Cochrane Database Syst Rev. 2002;2002(2):CD001357. doi: 10.1002/14651858.CD001357. Cochrane Database Syst Rev. 2002. PMID: 12076408 Free PMC article.
-
Atypical antipsychotics for psychosis in adolescents.Cochrane Database Syst Rev. 2013 Oct 15;2013(10):CD009582. doi: 10.1002/14651858.CD009582.pub2. Cochrane Database Syst Rev. 2013. PMID: 24129841 Free PMC article.
-
Clozapine dose for schizophrenia.Cochrane Database Syst Rev. 2017 Jun 14;6(6):CD009555. doi: 10.1002/14651858.CD009555.pub2. Cochrane Database Syst Rev. 2017. PMID: 28613395 Free PMC article.
Cited by
-
Current approaches to treatments for schizophrenia spectrum disorders, part I: an overview and medical treatments.Neuropsychiatr Dis Treat. 2013;9:1311-32. doi: 10.2147/NDT.S37485. Epub 2013 Sep 11. Neuropsychiatr Dis Treat. 2013. PMID: 24049446 Free PMC article. Review.
-
Adverse effects of atypical antipsychotics in the elderly: a review.Drugs Aging. 2006;23(12):937-56. doi: 10.2165/00002512-200623120-00002. Drugs Aging. 2006. PMID: 17154659 Review.
-
Sexual dysfunction and other prolactin-related side effects of antipsychotic drugs in schizophrenia: Protocol for a systematic review with single-arm, pairwise, and network meta-analyses of randomized controlled trials and non-randomized studies.F1000Res. 2025 Jun 19;13:973. doi: 10.12688/f1000research.154742.3. eCollection 2024. F1000Res. 2025. PMID: 40474917 Free PMC article.
-
Haloperidol (oral) versus olanzapine (oral) for people with schizophrenia and schizophrenia-spectrum disorders.Cochrane Database Syst Rev. 2024 Jul 3;7(7):CD013425. doi: 10.1002/14651858.CD013425.pub2. Cochrane Database Syst Rev. 2024. PMID: 38958149 Free PMC article.
-
Straightforward synthesis of N-arylindoles via one-pot Fischer indolisation-indole N-arylation.RSC Adv. 2023 May 26;13(23):15993-15997. doi: 10.1039/d3ra02658b. eCollection 2023 May 22. RSC Adv. 2023. PMID: 37250219 Free PMC article.
References
References to studies included in this review
Daniel 1998 {published data only}
-
- Chang R, Swann AC, Becker L, Wozniak PG, McCarthy BG. A one‐year, controlled cost‐effectiveness study comparing sertindole and haloperidol in stable schizophrenic patients. Biological Psychiatry 1998;43(suppl 1S):117S.
-
- Daniel DG, Wozniak P, Mack RJ, McCarthy BG. Long‐term efficacy and safety comparison of sertindole and haloperidol in the treatment of schizophrenia. Psychopharmacology Bulletin 1998;34(1):61‐9. - PubMed
-
- Foley S, Wozniak P, Silber C, Mack R. A multi‐center, one year, haloperidol controlled trial assessing the long term safety, efficacy and quality of life of sertindole in stable schizophrenic patients. 10th ECNP (European College of Neuropsychopharmacology) Congress, Vienna, Austria. 1997.
-
- Krystal J, D'Souza DC, Holgate K, Staser J, Silber C, Mack R. A multi‐centre, one year, haloperidol controlled trial assessing the long term safety, efficacy and quality of life of sertindole in stable schizophrenic patients. 35th American College of Neuropsychopharmacology (ACNP) Annual Meeting Abstracts, December 9‐13, 1996. 1996:252.
-
- Swann A, Holgate K, Staser J. Silber CMD, Mack R. A multi‐center, one year, haloperidol controlled trial assessing the long term safety, efficacy and quality of life of sertindole in stable schizophrenic patients. Schizophrenia Research (Special Issue ‐ The VIth International Congress on Schizophrenia Research, Colorado Springs, Colorado, USA) 1997;24:203.
Hale 2000 {published data only}
-
- Hale A, Wehnert A. Dose ranging study comparing four doses of sertindole and one dose of haloperidol in schizophrenic patients. XXth Collegium Internationale Neuro psychopharmacologicum. Melbourne,‐Australia, 1996.
-
- Hale A, Azorin J‐M, Kasper S, Maier W, Syvalahti E, Burght M, Sloth‐Nielsen M, Wehnert A. Sertindole improves both the positive and negative symptoms of schizophrenia: Results of a phase III trial. International Journal of Psychiatry in Clinical Practice 2000;4(1):55‐62. - PubMed
-
- Hale A, Azorin J‐M, Kasper S, Maier W, Syvalahti E, Burght M, Sloth‐Nielsen M, Wehnert A. Sertindole is associated with a low level of extrapyramidal symptoms in schizophrenic patients: Results of a phase III trial. International Journal of Psychiatry in Clinical Practice 2000;4:47‐54. - PubMed
-
- Hale A, Burght M, Wehnert A, et al. A European dose‐range study comparing the efficacy, tolerability and safety of four doses of Sertindole and one dose of haloperidol in schizophrenic patients (abstract). 35th American College of Neuropshchopharmacology Annual Meeting. San Juan, Puerto Rico: American College of Neuropsychopharmacology, Nashville (TN), 1996:252.
-
- Wehnert A, Mack R, Silber C, et al. Sertindole: an overview of the North American and European clinical data. Biological Psychiatry 1997;42(Suppl).
Van Kammen 1996 {published data only}
-
- Grebb JA, Sebree T, Schmitz P, Kashkin K, the M92‐762 Sertindole Research Group. A placebo‐controlled trial of sertindole in schizophrenia. Neuropsychopharmacology 1993;9(Suppl):119‐20.
-
- Martin P, Grebb JA, Schmitz P, et al. Efficacy and safety of sertindole in two double‐blind, placebo‐controlled trials of schizophrenic patients. Schizophrenia Research 1994;11(2):107.
-
- Potkin S, Schulz S, Mack R, Zborowski J, Morris D, Sebree T, et al. Efficacy and safety of sertindole in two double‐blind, placebo‐controlled trials of schizophrenic patients. Seventh Biennial Schizophrenia Winter Workshop, Diablerets, Switzerland, 1994. 1994.
-
- Targum S, Wallin B. Efficacy and safety of sertindole in two double‐blind, placebo‐controlled trials of schizophrenic patients. 8th ECNP (European College of Neuropsychopharmacology) Congress, Venice, Italy. 1995.
-
- Kammen DP, McEvoy JP, Tagum SD, Kardatzke D, Sebree T. A randomized, controlled, dose‐ranging trial of sertindole in patients with schizophrenia. Psychopharmacology 1996;124:168‐75. - PubMed
References to studies excluded from this review
Bark 1997 {published data only}
-
- Bark N, Moynihan N, Silva‐Siegel DD. Sertindole's improvement of negative symptoms, is it independent of extra‐pyramidal symptoms, depression and positive symptoms?. Schizophrenia Research (Special Issue ‐ The VIth International Congress on Schizophrenia Research, Colorado Springs, Colorado, USA) 1997;24:201.
Barnes 1998 {published data only}
-
- Barnes TRE, McPhillips MA. Novel antipsychotics, extrapyramidal side effects and tardive dyskinesia. International Clinical Psychopharmacology 1998;13(Suppl 3):S49‐S57. - PubMed
Borison 1995 {published data only}
-
- Borison RL. Clinical efficacy of serotonin‐dopamine antagonists relative to classic neuroleptics. Journal of Clinical Psychopharmacology 1995;15(1, Suppl 1):24S‐9S. - PubMed
Braus 1996 {published data only}
-
- Braus A, Nabulsi AA, Mack RJ, Holgate KL. Reduction of hospital days in sertindole‐treated patients ‐ one year findings. Schizophrenia 1996: Breaking down the Barriers, 4th International Conference, Vancouver, BC, Canada. 1996.
-
- Ereshefsky L, Nabulsi A, Silber C, Mack R. Reduction of hospital days in sertindole treated patients: one year findings. Schizophrenia Research (Special Issue) ‐ The VIth Internantional Congress on Schizophrenia Research, Colorado Springs, Colorado, USA 1997;24:201.
-
- Ereshefsky L, Targum S, Nabulsi A, Silber C, Mack R. Reduction of hospital days in sertindole treated patients ‐ one year findings. 35th American College of Neuropshchopharmacology Annual Meeting, San Juan, Puerto Rico. 1996:264.
-
- Nabulsi AA, Mack RJ, Sebree TB, Copeland LF, Holgate KL, Wallin BA. Reduction of hospital days in sertindole‐treated patients ‐ one‐year findings. American Psychiatric Association, 149th Annual Meeting. 1996.
-
- Ramirez L, Nabulsi AA, Mack RJ, Sebree TB, Copeland LA, Holgate KL. Reduction of hospital days in sertindole treated patients. Xth World Congress of Psychiatry, Madrid, Spain. 1996.
Brown 1997 {published data only}
-
- Brown GR, Radford JM. Sertindole hydrochloride: a novel antipsychotic medication with a favorable side effect profile. Southern Medical Journal 1997;90:691‐3. - PubMed
Buchsbaum 1997 {published data only}
-
- Buchsbaum M, Hazlett E, Bark N, Gupta A, Fallon J, Guich S, et al. Positron emission tomography in schizophrenics treated with atypical and typical neuroleptics. Schizophrenia Research (Special Issue ‐ The VIth International Congress on Schizophrenia Research, Colorado Springs, Colorado, USA) 1997;24:163.
-
- Buchsbaum M, Hazlett E, Haznedar M. Positron emission tomography in schizophrenics treated with sertindole and haloperidol. XXth Collegium Internationale Neuro‐psychopharmacologium (CINP), Melbourne, Australia. 1996.
-
- Buchsbaum M, Hazlett E, Haznedar M. Positron emission tomography in schizophrenics treated with sertindole and haloperidol. Xth World Congress of Psychiatry, Madrid, Spain, August 23‐28. 1996.
-
- Buchsbaum M, Hazlett E, Haznedar M, Mack R, Sebree T. Positron emission tomography in schizophrenic patients treated with sertindole and haloperidol. 9th ECNP (European College of Neuropsychopharmacology) Congress, Amsterdam, The Netherlands. 1996.
Daniel 1995 {published data only}
-
- Daniel D, Schmitz P, Staser J, Holgate K, Sebree T, Graves M. Two open‐label long‐term, safety studies of sertindole. American Psychiatric Association, 149th Annual Meeting, New York, NY, USA. 1996.
-
- Daniel D, Staser J, Schmiz P, Sebree T, Wallin B. Two open‐label, long‐term safety studies of sertindole. 8th ECNP (European College of Neuropsychopharmacology) Congress, Venice, Italy. 1995.
Dunn 1996 {published data only}
-
- Dunn CJ, Fitton A. Sertindole. CNS Drugs 1996;5:224‐30.
Geracioti 1998 {published data only}
-
- Geracioti TD, Parker S, Lowther NB, Wortman M, Richtand NM. A case of treatment‐refractory psychosis responsive to sertindole. Schizophrenia Research 1998;30(1):105‐8. - PubMed
Granneman 1997 {published data only}
-
- Granneman R, Wozniak P, Tran‐Johnson T, Silber C, Mack R. Population pharmacokinetics of sertindole during long‐term treatment of patients with schizophrenia. Schizophrenia Research (Special Issue ‐ The VIth Internantional Congress on Schizophrenia Research, Colorado Springs, Colorado, USA) 1997;24:202.
Hale 1998 {published data only}
-
- Hale A. A review of the safety and tolerability of sertindole. International Clinical Psychopharmacology 1998;13(suppl 3):S65‐S70. - PubMed
Janicak 1996 {published data only}
-
- Janicak PG, Davis JM. Antipsychotic dosing strategies in acute schizophrenia. International Clinical Psychopharmacology 1996;11(Suppl 2):35‐40. - PubMed
Mack 1997 {published data only}
-
- Mack R, Driscoll R, Silber C. The long term cardiovascular safety of sertindole. 10th ECNP (European College of Neuropsychopharmacology) Congress, Vienna, Austria. 1997.
Moeller 1998 {published data only}
-
- Moeller H‐J. Novel antipsychotics and negative symptoms. International Clinical Psychopharmacology 1998;13(Suppl 3):S43‐7. - PubMed
Pezawas 1997 {published data only}
-
- Pezawas L, Kufferle B, Barnas C, Wolf R, Kasper S. Sertindole in clinical practice. 10th ECNP (European College of Neuropsychopharmacology) Congress, Vienna, Austria. 1997.
Potkin 1994 {published data only}
-
- Kane JM. Sertindole: A review of clinical efficacy. International Clinical Psychopharmacology 1998;vol 13(suppl 3):S59‐S64. - PubMed
-
- Kasper S. Negative symptoms and sertindole. 9th ECNP (European College of Neuropsychopharmacology) Congress, Amsterdam, The Netherlands. 1996.
-
- Potkin S, Schulz S, Mack R, Zborowski J, Morris D, Sebree T, et al. Efficacy and safety of sertindole in two double‐blind, placebo‐controlled trials of schizophrenic patients. Seventh Biennial Schizophrenia Winter Workshop, Diablerets, Switzerland. 1994.
-
- Potkin SG, Zborowski J, Wu JC, Mack RJ, Sebree TS, Wallin A. Brain imaging to determine the effects of sertindole in schizophrenic patients. American Psychiatric Association, 149th Annual Meeting. 1996.
-
- Schulz S, Mack R, Zborowski J, Morris D, Sebree T, Wallin B. Efficacy, safety and dose response of three doses of sertindole and three doses of haldol in schizophrenic patients. Schizophrenia Research (8th Biennial Winter Workshop on Schizophrenia). Switzerland, 1996; Vol. 18:133.
Rasmussen 1997 {published data only}
-
- Rasmussen JGC. Drug treatments for schizophrenia ‐ past, present, and future. International Journal of Psychiatry in Clinical Practice 1997;1(4):227‐30. - PubMed
Sebree 1996 {published data only}
-
- Sebree TB, Cutler NR, Sramek JJ, Mack RJ, O'Neil JM. Rapid dose escalating PK study of sertindole in schizophrenics. Xth World Congress of Psychiatry, Madrid, Spain. 1996.
Tamminga 1997 {published data only}
-
- Tamminga CA, Mack RJ, Granneman R, Silber CJ, Kashkin KB. Sertindole in the treatment of psychosis in schizophrenia: efficacy and safety. International Clinical Psychopharmacology 1997;12(Suppl 1):S29‐S35. - PubMed
Wehnert 1997 {published data only}
-
- Wehnert A. The European post‐marketing observational serdolect (EPOS) project: increasing our understanding of schizophrenia therapy. International Clinical Psychopharmacology 1998;13(Suppl 3):S27‐S30. - PubMed
-
- Wehnert A, Hale A, Kasper S, Moeller H‐J, Campbell R, Stilwell C, et al. EPOS: Increasing our understanding of the treatment of schizophrenia: start of a prospective referenced cohort study of sertindole in clinical practice. International Journal of Psychiatry in Clinical Practice 1997;1(3):197‐202. - PubMed
-
- Wehnert A, Hale A, Kasper SM, Iler HJ, Campbell R, Stilwell C, et al. The EPOS project: Increasing our understanding of schizophrenia therapy. Commencement of a prospective, referenced, cohort study of Sertindole in clinical practice. 10th ECNP (European College of Neuropsychopharmacology) Congress, Vienna, Austria. 1997.
Wehnert 1997b {published data only}
-
- Mack R, Foley S, Silber C. The action of sertindole on the negatve symptoms in schizophrenia. 10th ECNP (European College of Neuropsychopharmacology) Congress, Vienna, Austria. 1997.
-
- Wehnert A, Mack R, Stilwell C, Rasmussen C, Silber CH. Direct effect of sertindole on the primary negative symptoms of schizophrenia: a PATH analysis. Biological Psychiatry 1997;42(suppl 1):188.
Zimbroff 1997 {published data only}
-
- Baker RW, Mack R, Morris D, Sebree T, Kashkin K. The efficacy and safety of three doses of sertindole versus three doses of haloperidol in schizophrenic patients. 9th ECNP (European College of Neuropsychopharmacology) Congress, Amsterdam, The Netherlands. 1996.
-
- Baker RW, Mack RJ, Morris DD, Sebree T, Kashkin K. The efficacy and safety of three doses of sertindole versus three doses of haloperidol in schizophrenic patients. European Neuropsychopharmacology. 1996; Vol. 6, issue Suppl. 4.
-
- Daniel D. Sertindole therapy is effective in patients with schizophrenia. American Family Physician 1996;53(3):939‐40.
-
- Kasper S. Negative symptoms and sertindole. 9th ECNP (European College of Neuropsychopharmacology) Congress, Amsterdam, The Netherlands. 1996.
-
- Larson GL, Mack JR, Zborowski J, Morris DD, Sebree TB, Wallin BA. Three doses each of sertindole and haloperidol in schizophrenics. Xth World Congress of Psychiatry, Madrid, Spain. 1996.
References to studies awaiting assessment
Azorin, 2002 {published data only}
-
- Azorin JM, Toumi M, Sloth‐Nielsen M. Sertindole is well tolerated and superior to risperidone with respect to efficacy in patients with schizophrenia. European Neuropsychopharmacology 2002;12 (suppl. 3):S300.
Martin 1994 {published data only}
-
- Martin P, Grebb JA, Schmitz P, et al. Efficacy and safety of sertindole in two double‐blind, placebo‐controlled trials of schizophrenic patients. Schizophrenia Research 1994;11(2):11‐107.
McEvoy 1993 {published data only}
-
- McEvoy J, Borison R, Small J, Kammen DV, Meltzer H, Hamner M, Morris D, Shu V, Sebree T, Grebb J, Kashkin K. The efficacy and tolerability of sertindole in schizophrenic patients: a pilot, double‐blind, placebo‐controlled, dose‐ranging study. Schizophrenia Research 1993;9(2, 3):244.
Additional references
Adams 1999
-
- Adams CE, Gray R, Daniels J, Thornley B, Philpott H, Wahlbeck K. A survey of UK consultant psychiatrists' first and second choice drug treatments for schizophrenia. Unpublished report.
Altman 1996
APA 1997
-
- American Psychiatric Association. American Psychiatric Association Practice Guidelines. Practice guideline for the treatment of patients with schizophrenia. American Journal of Psychiatry 1997;154(4):1‐63. - PubMed
Barnes 1989
-
- Barnes TR. A rating scale for drug‐induced akathisia. British Journal of Psychiatry 1989;154:672‐6. - PubMed
Begg 1996
-
- Begg C, Cho M, Eastwood S, Horton R, Moher D, Olkin I, Pitkin R, Rennie D, Schulz KF, Simel D, Stroup DF. Improving the quality of randomized controlled trials. The CONSORT statement. JAMA 1996;276:637‐9. - PubMed
Buckley 1997
-
- Buckley PF. New dimensions in the pharmacologic treatment of schizophrenia and related psychoses. Journal of Clinical Pharmacology 1997;37:363‐78. - PubMed
Carpenter 1994
-
- Carpenter WT Jr, Buchanan RW. Schizophrenia. New England Journal of Medicine 1994;330:681‐90. - PubMed
Casey 1997
-
- Casey DE. The relationship of pharmacology to side effects. Journal of Clinical Psychiatry 1997;58(10):55‐62. - PubMed
Chalmers 1983
-
- Chalmers TC, Celano P, Sacks HS, Smith HJr. Bias in treatment assignment in controlled clinical trials. New England Journal of Medicine 1983;309:1358‐61. - PubMed
COSTART 1990
-
- US Food, Drug Administration. COSTART coding symbols for thesaurus of adverse reaction terms. 2. Rockville: Food and Drug Administration, 1990.
CWG 1998
-
- Collaborative Working Group on Clinical Trial Evaluations. Adverse effects of the atypical antipsychotics. Journal of Clinical Psychiatry 1998;59:17‐22. - PubMed
Duggan 1999
Egger 1997
Glazer 1997
-
- Glazer WM, Johnstone BM. Pharmacoeconomic evaluation of antipsychotic therapy for schizophrenia. Journal of Clinical Psychiatry 1997;58(10):50‐4. - PubMed
Guy 1976
-
- Guy W. Early clinical drug evaluation (ECDEU) assessment manual for psychopharmacology. National Institute of Mental Health Publication No.76‐338. Washington, DC: National Institute of Mental Health Publication, 1976:217‐22.
Kane 1996
-
- Kane JM. Factors which can make patients difficult to treat. British Journal of Psychiatry 1996;169(31):10‐4. - PubMed
Kay 1987
-
- Kay SR, Fiszbein A, Opler LA. The Positive and Negative Syndrome Scale (PANSS) for schizophrenia. Schizophrenia Bulletin 1987;13:261‐7. - PubMed
Kerwin 1994
-
- Kerwin RW. The new atypical antipsychotics. A lack of extrapyramidal side‐effects and new routes in schizophrenia research. British Journal of Psychiatry 1994;164:141‐8. - PubMed
Lundbeck Ltd. 1997
-
- Lundbeck Ltd. Serdolect (sertindole) product monograph. Lundbeck Ltd. 1997.
Meltzer 1996
-
- Meltzer HY. Cost‐effectiveness of clozapine treatment. Journal of Clinical Psychiatry. Monograph Series 1996;14:16‐7.
Moher 1998
-
- Moher D, Pham B, Jones A, Cook DJ, Jadad AR, Moher M, Tugwell P, Klassen TP. Does quality of reports of randomised trials affect estimates of intervention efficacy reported in meta‐analyses?. Lancet 1998;352:609‐13. - PubMed
Mulrow 1997
-
- Mulrow CD, Oxman AD (eds). Cochrane Collaboration Handbook [updated 1 March 1997]. Cochrane Database of Systematic Reviews 1997, Issue 1.
Overall 1986
-
- Overall JE, Gorham DR. The Brief Psychiatric Rating Scale. Psychological Reports 1962;10:799‐812.
RCP 1999
-
- Royal College of Psychiatrists. Guidelines on for the care of those with schizophrenia. In press 1999.
Schulz 1995
-
- Schulz KF, Chalmers I, Hayes RJ, Altman DG. Empirical evidence of bias: dimensions of methodological quality associated with estimates of treatment effects in controlled trials. JAMA 1995;273:408‐12. - PubMed
Silverstone 1995
-
- Silverstone T, Turner P. Drug treatment in psychiatry. 5th Edition. Cornwall: TJ Press, 1995.
Simpson 1970
-
- Simpson GM, Angus JWS. A rating scale for extrapyramidal side effects. Acta Psychiatria Scandinavica 1970;212:S11‐9. - PubMed
Verhagen 1999
-
- Verhagen AP, Bie RA, Lenssen AF, et al. Impact of quality items on study outcome: treatments in acute lateral ankle sprains. Quality assessment of randomised clinical trials. PhD Thesis. Maastricht, 1999:83‐94. - PubMed
Wood 1998
-
- Wood M. Review of the global schizophrenia market. Pharma Forum 1998;39:9.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

