De novo formation of left-right asymmetry by posterior tilt of nodal cilia
- PMID: 16035921
- PMCID: PMC1180513
- DOI: 10.1371/journal.pbio.0030268
De novo formation of left-right asymmetry by posterior tilt of nodal cilia
Abstract
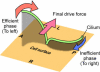
In the developing mouse embryo, leftward fluid flow on the ventral side of the node determines left-right (L-R) asymmetry. However, the mechanism by which the rotational movement of node cilia can generate a unidirectional flow remains hypothetical. Here we have addressed this question by motion and morphological analyses of the node cilia and by fluid dynamic model experiments. We found that the cilia stand, not perpendicular to the node surface, but tilted posteriorly. We further confirmed that such posterior tilt can produce leftward flow in model experiments. These results strongly suggest that L-R asymmetry is not the descendant of pre-existing L-R asymmetry within each cell but is generated de novo by combining three sources of spatial information: antero-posterior and dorso-ventral axes, and the chirality of ciliary movement.
Figures

References
-
- Hamada H, Meno C, Watanabe D, Saijoh Y. Establishment of vertebrate left-right asymmetry. Nat Rev Genet. 2002;3:103–113. - PubMed
-
- Nonaka S, Tanaka Y, Okada Y, Takeda S, Harada A. Randomization of left-right asymmetry due to loss of nodal cilia generating leftward flow of extraembryonic fluid in mice lacking KIF3B motor protein. Cell. 1998;95:829–837. - PubMed
-
- Nonaka S, Shiratori H, Saijoh Y, Hamada H. Determination of left-right patterning of the mouse embryo by artificial nodal flow. Nature. 2002;418:96–99. - PubMed
-
- Tabin CJ, Vogan KJ. A two-cilia model for vertebrate left-right axis specification. Genes Dev. 2003;17:1–6. - PubMed
-
- McGrath J, Somlo S, Makova S, Tian X, Brueckner M. Two populations of node monocilia initiate left-right asymmetry in the mouse. Cell. 2003;114:61–73. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources

