Brain tumor tropism of transplanted human neural stem cells is induced by vascular endothelial growth factor
- PMID: 16036113
- PMCID: PMC1501284
- DOI: 10.1593/neo.04781
Brain tumor tropism of transplanted human neural stem cells is induced by vascular endothelial growth factor
Abstract
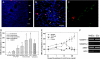
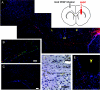
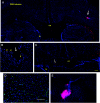
The transplantation of neural stem cells (NSCs) offers a new potential therapeutic approach as a cell-based delivery system for gene therapy in brain tumors. This is based on the unique capacity of NSCs to migrate throughout the brain and to target invading tumor cells. However, the signals controlling the targeted migration of transplanted NSCs are poorly defined. We analyzed the in vitro and in vivo effects of angiogenic growth factors and protein extracts from surgical specimens of brain tumor patients on NSC migration. Here, we demonstrate that vascular endothelial growth factor (VEGF) is able to induce a long-range attraction of transplanted human NSCs from distant sites in the adult brain. Our results indicate that tumor-upregulated VEGF and angiogenic-activated microvasculature are relevant guidance signals for NSC tropism toward brain tumors.
Figures
References
-
- Kleihues P, Cavenee WK. Pathology and Genetics of Tumors of the Central Nervous System International Agency for Research on Cancer Press, Lyon. 2000.
-
- Benedetti S, Pirola B, Pollo B, Magrassi L, Bruzzone MG, Rigamonti D, Galli R, Selleri S, Di Meco F, De Fraja C, et al. Gene therapy of experimental brain tumors using neural progenitor cells. Nat Med. 2000;6:447–450. - PubMed
-
- Ehtesham M, Kabos P, Kabosova A, Neuman T, Black KL, Yu JS. The use of interleukin 12-secreting neural stem cells for the treatment of intracranial glioma. Cancer Res. 2002;62:5657–5663. - PubMed
-
- Staflin K, Honeth G, Kalliomaki S, Kjellman C, Edvardsen K, Lindvall M. Neural progenitor cell lines inhibit rat tumor growth in vivo. Cancer Res. 2004;64:5347–5354. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
