Alk3/Bmpr1a receptor is required for development of the atrioventricular canal into valves and annulus fibrosus
- PMID: 16037571
- PMCID: PMC2950023
- DOI: 10.1161/01.RES.0000177862.85474.63
Alk3/Bmpr1a receptor is required for development of the atrioventricular canal into valves and annulus fibrosus
Abstract
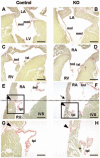
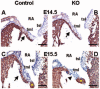
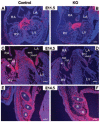
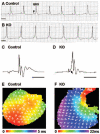
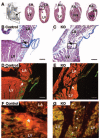
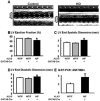
Endocardial cushions are precursors of mature atrioventricular (AV) valves. Their formation is induced by signaling molecules originating from the AV myocardium, including bone morphogenetic proteins (BMPs). Here, we hypothesized that BMP signaling plays an important role in the AV myocardium during the maturation of AV valves from the cushions. To test our hypothesis, we used a unique Cre/lox system to target the deletion of a floxed Alk3 allele, the type IA receptor for BMPs, to cardiac myocytes of the AV canal (AVC). Lineage analysis indicated that cardiac myocytes of the AVC contributed to the tricuspid mural and posterior leaflets, the mitral septal leaflet, and the atrial border of the annulus fibrosus. When Alk3 was deleted in these cells, defects were seen in the same leaflets, ie, the tricuspid mural leaflet and mitral septal leaflet were longer, the tricuspid posterior leaflet was displaced and adherent to the ventricular wall, and the annulus fibrosus was disrupted resulting in ventricular preexcitation. The defects seen in mice with AVC-targeted deletion of Alk3 provide strong support for a role of Alk3 in human congenital heart diseases, such as Ebstein's anomaly. In conclusion, our mouse model demonstrated critical roles for Alk3 signaling in the AV myocardium during the development of AV valves and the annulus fibrosus.
Figures

References
-
- Hoffman J. Incidence of congenital heart disease: II. Prenatal incidence. Pediatr Cardiol. 1995;16:155–165. - PubMed
-
- Eisenberg LM, Markwald RR. Molecular regulation of atrioventricular valvuloseptal morphogenesis. Circ Res. 1995;77:1–6. - PubMed
-
- Markwald R, Eisenberg C, Eisenberg L, Trusk T, Sugi Y. Epithelial-mesenchymal transformations in early avian heart development. Acta Anat (Basel) 1996;156:173–186. - PubMed
-
- Schroeder JA, Jackson LF, Lee DC, Camenisch TD. Form and function of developing heart valves: coordination by extracellular matrix and growth factor signaling. J Mol Med. 2003;81:392–403. - PubMed
-
- Barnett JV, Desgrosellier JS. Early events in valvulogenesis: a signaling perspective. Birth Defects Res C Embryo Today. 2003;69:58–72. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 HL033107/HL/NHLBI NIH HHS/United States
- R01 HL064757/HL/NHLBI NIH HHS/United States
- 1P01HL69020/HL/NHLBI NIH HHS/United States
- R01 HL076751/HL/NHLBI NIH HHS/United States
- P01 HL069020/HL/NHLBI NIH HHS/United States
- HL55373/HL/NHLBI NIH HHS/United States
- P01 HL059139/HL/NHLBI NIH HHS/United States
- P01 AR042919/AR/NIAMS NIH HHS/United States
- WT_/Wellcome Trust/United Kingdom
- R01 AG014121/AG/NIA NIH HHS/United States
- AR42919/AR/NIAMS NIH HHS/United States
- 2R01HL33107/HL/NHLBI NIH HHS/United States
- R01 HL055373/HL/NHLBI NIH HHS/United States
- 2R01AG14121/AG/NIA NIH HHS/United States
- 2P01HL59139/HL/NHLBI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

