Spatiotemporal mapping of cortical activity accompanying voluntary movements using an event-related beamforming approach
- PMID: 16037985
- PMCID: PMC6871358
- DOI: 10.1002/hbm.20178
Spatiotemporal mapping of cortical activity accompanying voluntary movements using an event-related beamforming approach
Abstract
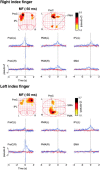
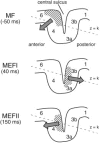
We describe a novel spatial filtering approach to the localization of cortical activity accompanying voluntary movements. The synthetic aperture magnetometry (SAM) minimum-variance beamformer algorithm was used to compute spatial filters three-dimensionally over the entire brain from single trial neuromagnetic recordings of subjects performing self-paced index finger movements. Images of instantaneous source power ("event-related SAM") computed at selected latencies revealed activation of multiple cortical motor areas prior to and following left and right index finger movements in individual subjects, even in the presence of low-frequency noise (e.g., eye movements). A slow premovement motor field (MF) reaching maximal amplitude approximately 50 ms prior to movement onset was localized to the hand area of contralateral precentral gyrus, followed by activity in the contralateral postcentral gyrus at 40 ms, corresponding to the first movement-evoked field (MEFI). A novel finding was a second activation of the precentral gyrus at a latency of approximately 150 ms, corresponding to the second movement-evoked field (MEFII). Group averaging of spatially normalized images indicated additional premovement activity in the ipsilateral precentral gyrus and the left inferior parietal cortex for both left and right finger movements. Weaker activations were also observed in bilateral premotor areas and the supplementary motor area. These results show that event-related beamforming provides a robust method for studying complex patterns of time-locked cortical activity accompanying voluntary movements, and offers a new approach for the localization of multiple cortical sources derived from neuromagnetic recordings in single subject and group data.
Copyright 2005 Wiley-Liss, Inc.
Figures
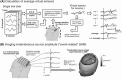
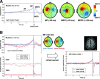
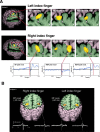
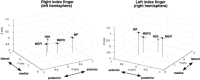
References
-
- Alkadhi H, Crelier GR, Boendermaker SH, Hepp‐Reymond MC, Kollias SS (2002): Somatotopy in the ipsilateral primary motor cortex. Neuroreport 13: 2065–2070. - PubMed
-
- Allison JD, Meador KJ, Loring DW, Figueroa RE, Wright JC (2000): Functional MRI cerebral activation and deactivation during finger movement. Neurology 54: 135–142. - PubMed
-
- Andersen RA, Snyder LH, Bradley DC, Xing J (1997): Multimodal representation of space in the posterior parietal cortex and its use in planning movements. Annu Rev Neurosci 20: 303–330. - PubMed
-
- Bakhtazad L, Gaetz W, Cheyne D (2004): High resolution neuromagnetic imaging using minimum‐variance beamforming reveals multiple generators of movement‐evoked fields. In: Halgren E, Ahlfors S, Hamalainen M, Cohen D, editors. Proc 14th Int Conf Biomagnetism. Boston: Biomag 2004. p 723.
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources

