Multiple requirements for Hes 1 during early eye formation
- PMID: 16038893
- PMCID: PMC4128414
- DOI: 10.1016/j.ydbio.2005.06.010
Multiple requirements for Hes 1 during early eye formation
Abstract
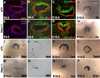
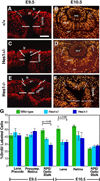
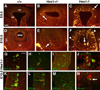
During embryogenesis, multiple developmental processes are integrated through their precise temporal regulation. Hes1 is a transcriptional repressor that regulates the timing of mammalian retinal neurogenesis. However, roles for Hes1 in early eye development have not been well defined. Here, we show that Hes1 is expressed in the forming lens, optic vesicle, cup, and pigmented epithelium and is necessary for proper growth, morphogenesis, and differentiation of these tissues. Because Hes1 is required throughout the eye, we investigated its interaction with Pax6. Hes1-Pax6 double mutant embryos are eyeless suggesting these genes are coordinately required for initial morphogenesis and outgrowth of the optic vesicle. In Hes1 mutants, Math5 expression is precocious along with retinal ganglion cell, amacrine, and horizontal neuron formation. In contrast to apparent cooperativity between Pax6 and Hes1 during morphogenesis, each gene regulates Math5 and RGC genesis independently. Together, these studies demonstrate that Hes1, like Pax6, simultaneously regulates multiple developmental processes during optic development.
Figures




References
-
- Ahmad I, Dooley CM, Polk DL. Delta-1 is a regulator of neurogenesis in the vertebrate retina. Dev. Biol. 1997;185:92–103. - PubMed
-
- Akagi T, Inoue T, Miyoshi G, Bessho Y, Takahashi M, Lee JE, Guillemot F, Kageyama R. Requirement of multiple basic helix-loop-helix genes for retinal neuronal subtype specification. J. Biol. Chem. 2004;279:28492–28498. - PubMed
-
- Alexiades MR, Cepko CL. Subsets of retinal progenitors display temporally regulated and distinct biases in the fates of their progeny. Development. 1997;124:1119–1131. - PubMed
-
- Andreazzoli M, Gestri G, Cremisi F, Casarosa S, Dawid IB, Barsacchi G. Xrx1 controls proliferation and neurogenesis in Xenopus anterior neural plate. Development. 2003;130:5143–5155. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

