Abeta42 is essential for parenchymal and vascular amyloid deposition in mice
- PMID: 16039562
- PMCID: PMC1373682
- DOI: 10.1016/j.neuron.2005.06.030
Abeta42 is essential for parenchymal and vascular amyloid deposition in mice
Abstract
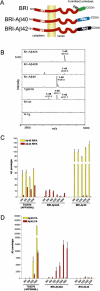
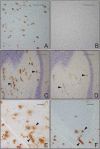
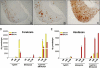
Considerable circumstantial evidence suggests that Abeta42 is the initiating molecule in Alzheimer's disease (AD) pathogenesis. However, the absolute requirement for Abeta42 for amyloid deposition has never been demonstrated in vivo. We have addressed this by developing transgenic models that express Abeta1-40 or Abeta1-42 in the absence of human amyloid beta protein precursor (APP) overexpression. Mice expressing high levels of Abeta1-40 do not develop overt amyloid pathology. In contrast, mice expressing lower levels of Abeta1-42 accumulate insoluble Abeta1-42 and develop compact amyloid plaques, congophilic amyloid angiopathy (CAA), and diffuse Abeta deposits. When mice expressing Abeta1-42 are crossed with mutant APP (Tg2576) mice, there is also a massive increase in amyloid deposition. These data establish that Abeta1-42 is essential for amyloid deposition in the parenchyma and also in vessels.
Figures


References
-
- Bales KR, Verina T, Cummins DJ, Du Y, Dodel RC, Saura J, Fishman CE, DeLong CA, Piccardo P, Petegnief V, et al. Proc. Natl. Acad. Sci. USA. Vol. 96. 1999. Apolipoprotein E is essential for amyloid deposition in the APP(V717F) transgenic mouse model of Alzheimer's disease; pp. 15233–15238. - PMC - PubMed
-
- Borchelt DR, Davis J, Fischer M, Lee MK, Slunt HH, Ratovitsky T, Regard J, Copeland NG, Jenkins NA, Sisodia SS, Price DL. A vector for expressing foreign genes in the brains and hearts of transgenic mice. Genet. Anal. 1996;13:159–163. - PubMed
-
- Borchelt DR, Ratovitski T, van Lare J, Lee MK, Gonzales V, Jenkins NA, Copeland NG, Price DL, Sisodia SS. Accelerated amyloid deposition in the brains of transgenic mice coexpressing mutant presenilin 1 and amyloid precursor proteins. Neuron. 1997;19:939–945. - PubMed
-
- Chishti MA, Yang DS, Janus C, Phinney AL, Horne P, Pearson J, Strome R, Zuker N, Loukides J, French J, et al. Early-onset amyloid deposition and cognitive deficits in transgenic mice expressing a double mutant form of amyloid precursor protein 695. J. Biol. Chem. 2001;276:21562–21570. - PubMed
-
- Farris W, Mansourian S, Chang Y, Lindsley L, Eckman EA, Frosch MP, Eckman CB, Tanzi RE, Selkoe DJ, Guenette S. Insulin-degrading enzyme regulates the levels of insulin, amyloid beta-protein, and the beta-amyloid precursor protein intra-cellular domain in vivo. Proc. Natl. Acad. Sci. USA. 2003;100:4162–4167. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
