Reflexes evoked by electrical stimulation of afferent axons in the pudendal nerve under empty and distended bladder conditions in urethane-anesthetized rats
- PMID: 16039722
- PMCID: PMC3119341
- DOI: 10.1016/j.jneumeth.2005.06.002
Reflexes evoked by electrical stimulation of afferent axons in the pudendal nerve under empty and distended bladder conditions in urethane-anesthetized rats
Abstract
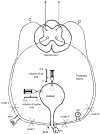
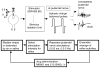
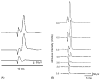
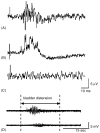
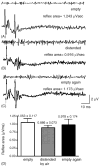
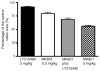
This study examined reflex mechanisms that mediate urinary bladder and external urethral sphincter (EUS) coordination in female Sprague-Dawley urethane-anesthetized rats under empty and distended bladder conditions. The bladder was distended either by a small balloon or a saline filled catheter inserted through the body of the bladder. Stimulation of the entire pudendal nerve elicited short latency (8-12 ms) responses in the EUS and short (3-8 ms) and long latency responses (16-20 ms) in contralateral pudendal nerve. The long latency pudendal-pudendal reflex was reduced by 36.7% in area during bladder distension with the balloon catheter. However, there was no significant change in the area of pudendal-EUS reflex during bladder distension. Peak amplitudes of both reflexes were reduced 32% by bladder distension. The effects of glutamatergic receptor antagonists on the reflexes were also examined. MK 801 (0.3-5mg/kg, i.v.), an N-methyl-d-aspartate glutamatergic receptor antagonist, markedly depressed the pudendal-pudendal reflex, but LY 215490 (3mg/kg, i.v.), an alpha-amino-5-methyl isoxazole-4-propionate antagonist, had a minimal inhibitory effect. Both glutamatergic receptor antagonists significantly suppressed the pudendal-EUS reflex. These results indicate that the EUS is innervated by multiple pathways and that glutamatergic excitatory transmission is important in the neural mechanisms underlying bladder-sphincter coordination in the rat.
Figures
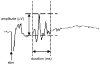


References
-
- Boggs JW, Wenzel BJ, Gustafson KJ, Grill WM. Spinal micturition reflex mediated by afferents in the deep perineal nerve. J Neurophysiol. 2005;93:2688–97. - PubMed
-
- de Groat WC. Anatomy and physiology of the lower urinary tract. Urol Clin North Am. 1993;20:383–401. - PubMed
-
- de Groat WC, Booth AM, Yoshimura N. Neurophysiology of micturition and its modification in animal models of humane disease. In: Maggi CA, editor. The autonomic nervous system: nervous control of the urogenital system. Vol. 3. London, UK: Harwood Academic Publishers; 1993. pp. 227–89.
-
- Jezernik S, Craggs M, Grill WM, Creasey G, Rijkhoff NJ. Electrical stimulation for the treatment of bladder dysfunction: current status and future possibilities. Neurol Res. 2002;24:413–30. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

