Autophagic nutrient recycling in Arabidopsis directed by the ATG8 and ATG12 conjugation pathways
- PMID: 16040659
- PMCID: PMC1183398
- DOI: 10.1104/pp.105.060673
Autophagic nutrient recycling in Arabidopsis directed by the ATG8 and ATG12 conjugation pathways
Abstract
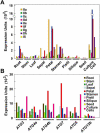
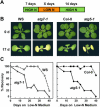
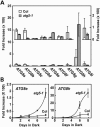
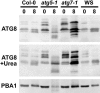
Autophagy is an important mechanism for nonselective intracellular breakdown whereby cytosol and organelles are encapsulated in vesicles, which are then engulfed and digested by lytic vacuoles/lysosomes. In yeast, this encapsulation employs a set of autophagy (ATG) proteins that direct the conjugation of two ubiquitin-like protein tags, ATG8 and ATG12, to phosphatidylethanolamine and the ATG5 protein, respectively. Using an Arabidopsis (Arabidopsis thaliana) atg7 mutant unable to ligate either tag, we previously showed that the ATG8/12 conjugation system is important for survival under nitrogen-limiting growth conditions. By reverse-genetic analyses of the single Arabidopsis gene encoding ATG5, we show here that the subpathway that forms the ATG12-ATG5 conjugate also has an essential role in plant nutrient recycling. Similar to plants missing ATG7, those missing ATG5 display early senescence and are hypersensitive to either nitrogen or carbon starvation, which is accompanied by a more rapid loss of organellar and cytoplasmic proteins. Multiple ATG8 isoforms could be detected immunologically in seedling extracts. Their abundance was substantially elevated in both the atg5 and atg7 mutants, caused in part by an increase in abundance of several ATG8 mRNAs. Using a green fluorescent protein-ATG8a fusion in combination with concanamycin A, we also detected the accumulation of autophagic bodies inside the vacuole. This accumulation was substantially enhanced by starvation but blocked in the atg7 background. The use of this fusion in conjunction with atg mutants now provides an important marker to track autophagic vesicles in planta.
Figures






Similar articles
-
The ATG12-conjugating enzyme ATG10 Is essential for autophagic vesicle formation in Arabidopsis thaliana.Genetics. 2008 Mar;178(3):1339-53. doi: 10.1534/genetics.107.086199. Epub 2008 Feb 3. Genetics. 2008. PMID: 18245858 Free PMC article.
-
ATG8 lipidation and ATG8-mediated autophagy in Arabidopsis require ATG12 expressed from the differentially controlled ATG12A AND ATG12B loci.Plant J. 2010 May;62(3):483-93. doi: 10.1111/j.1365-313X.2010.04166.x. Epub 2010 Feb 3. Plant J. 2010. PMID: 20136727
-
The ATG1/ATG13 protein kinase complex is both a regulator and a target of autophagic recycling in Arabidopsis.Plant Cell. 2011 Oct;23(10):3761-79. doi: 10.1105/tpc.111.090993. Epub 2011 Oct 7. Plant Cell. 2011. PMID: 21984698 Free PMC article.
-
Two ubiquitin-like conjugation systems that mediate membrane formation during autophagy.Essays Biochem. 2013;55:39-50. doi: 10.1042/bse0550039. Essays Biochem. 2013. PMID: 24070470 Review.
-
The Atg8 and Atg12 ubiquitin-like conjugation systems in macroautophagy. 'Protein modifications: beyond the usual suspects' review series.EMBO Rep. 2008 Sep;9(9):859-64. doi: 10.1038/embor.2008.163. EMBO Rep. 2008. PMID: 18704115 Free PMC article. Review.
Cited by
-
Autophagy Plays Prominent Roles in Amino Acid, Nucleotide, and Carbohydrate Metabolism during Fixed-Carbon Starvation in Maize.Plant Cell. 2020 Sep;32(9):2699-2724. doi: 10.1105/tpc.20.00226. Epub 2020 Jul 2. Plant Cell. 2020. PMID: 32616663 Free PMC article.
-
Plant abiotic stress signaling.Plant Signal Behav. 2012 Nov;7(11):1450-5. doi: 10.4161/psb.21894. Epub 2012 Sep 18. Plant Signal Behav. 2012. PMID: 22990453 Free PMC article. Review.
-
How Lipids Contribute to Autophagosome Biogenesis, a Critical Process in Plant Responses to Stresses.Cells. 2021 May 21;10(6):1272. doi: 10.3390/cells10061272. Cells. 2021. PMID: 34063958 Free PMC article. Review.
-
Plant Autophagy: An Intricate Process Controlled by Various Signaling Pathways.Front Plant Sci. 2021 Sep 23;12:754982. doi: 10.3389/fpls.2021.754982. eCollection 2021. Front Plant Sci. 2021. PMID: 34630498 Free PMC article. Review.
-
HOOKLESS1 acetylates AUTOPHAGY-RELATED PROTEIN18a to promote autophagy during nutrient starvation in Arabidopsis.Plant Cell. 2023 Dec 21;36(1):136-157. doi: 10.1093/plcell/koad252. Plant Cell. 2023. PMID: 37823521 Free PMC article.
References
-
- Buchanan-Wollaston V, Page T, Harrison E, Breeze E, Lim PO, Nam HG, Lin JF, Wu SH, Swidzinski J, Ishizaki K, et al (2005) Comparative transcriptome analysis reveals significant differences in gene expression and signaling pathways between developmental and dark/starvation-induced senescence in Arabidopsis. Plant J 42: 567–585 - PubMed
-
- Clough SJ, Bent AF (1998) Floral dip: a simplified method for Agrobacterium-mediated transformation of Arabidopsis thaliana. Plant J 16: 735–743 - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
- Actions
- Actions
- Actions
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

