Docosahexaenoic acid: a positive modulator of Akt signaling in neuronal survival
- PMID: 16040805
- PMCID: PMC1182431
- DOI: 10.1073/pnas.0502903102
Docosahexaenoic acid: a positive modulator of Akt signaling in neuronal survival
Erratum in
- Proc Natl Acad Sci U S A. 2005 Sep 6;102(36):12997
Abstract
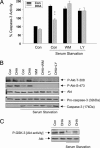
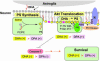
Phosphatidylinositol 3-kinase [PI (3)K]/Akt signaling is a critical pathway in cell survival. Here, we demonstrate a mechanism where membrane alteration by the n-3 fatty acid status affects Akt signaling, impacting neuronal survival. Docosahexaenoic acid (DHA), an n-3 polyunsaturated fatty acid highly enriched in neuronal membranes, promotes neuronal survival by facilitating membrane translocation/activation of Akt through its capacity to increase phosphatidylserine (PS), the major acidic phospholipid in cell membranes. The activation of PI (3)K and phosphatidylsinositol triphosphate formation were not affected by DHA, indicating that membrane interaction of Akt is the event responsible for the DHA effect. Docosapentaenoic acid, which replaces DHA during n-3 fatty acid deficiency, was less effective in accumulating PS and translocating Akt and thus less effective in preventing apoptosis. Consistently, in vivo reduction of DHA by dietary depletion of n-3 fatty acids decreased hippocampal PS and increased neuronal susceptibility to apoptosis in cultures. This mechanism may contribute to neurological deficits associated with n-3 fatty acid deficiency and support protective effects of DHA in pathological models such as brain ischemia or Alzheimer's disease.
Figures




References
-
- Dudek, H., Datta, S. R., Franke, T. F., Birnbaum, M. J., Yaou, R., Cooper, G. M., Segal, R. A., Kaplan, D. R. & Greenberg, M. E. (1997) Science 275, 661-665. - PubMed
-
- Segal, R. A. (2003) Annu. Rev. Neurosci. 26, 299-330. - PubMed
-
- Salem, N., Jr., Kim, H.-Y. & Yergey, J. A. (1986) in Health Effects of Polyunsaturated Fatty Acids in Seafoods, eds. Simopoulos, A. P. & Kifer, R. R. (Academic, New York), pp. 263-317.
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

