An ABC transporter containing a forkhead-associated domain interacts with a serine-threonine protein kinase and is required for growth of Mycobacterium tuberculosis in mice
- PMID: 16040957
- PMCID: PMC1201257
- DOI: 10.1128/IAI.73.8.4471-4477.2005
An ABC transporter containing a forkhead-associated domain interacts with a serine-threonine protein kinase and is required for growth of Mycobacterium tuberculosis in mice
Abstract
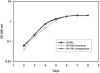
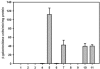
Forkhead-associated (FHA) domains are modular phosphopeptide recognition motifs with a striking preference for phosphothreonine-containing epitopes. FHA domains have been best characterized in eukaryotic signaling pathways but have been identified in six proteins in Mycobacterium tuberculosis, the causative organism of tuberculosis. One of these, coded by gene Rv1747, is an ABC transporter and the only one to contain two such modules. A deletion mutant of Rv1747 is attenuated in a mouse intravenous injection model of tuberculosis where the bacterial load of the mutant is 10-fold lower than that of the wild type in both lungs and spleen. In addition, growth of the mutant in mouse bone marrow-derived macrophages and dendritic cells is significantly impaired. In contrast, growth of this mutant in vitro was indistinguishable from that of the wild type. The mutant phenotype was lost when the mutation was complemented by the wild-type allele, confirming that it was due to mutation of Rv1747. Using yeast two-hybrid analysis, we have shown that the Rv1747 protein interacts with the serine-threonine protein kinase PknF. This interaction appears to be phospho-dependent since it is abrogated in a kinase-dead mutant and by mutations in the presumed activation loop of PknF and in the first FHA domain of Rv1747. These results demonstrate that the protein coded by Rv1747 is required for normal virulent infection by M. tuberculosis in mice and, since it interacts with a serine-threonine protein kinase in a kinase-dependent manner, indicate that it forms part of an important phospho-dependent signaling pathway.
Figures




References
-
- Av-Gay, Y., and M. Everett. 2000. The eukaryotic-like Ser/Thr protein kinases of Mycobacterium tuberculosis. Trends Microbiol. 8:238-244. - PubMed
-
- Boitel, B., M. Ortiz-Lombardia, R. Duran, F. Pompeo, S. T. Cole, C. Cervenansky, and P. M. Alzari. 2003. PknB kinase activity is regulated by phosphorylation in two Thr residues and dephosphorylation by PstP, the cognate phospho-Ser/Thr phosphatase, in Mycobacterium tuberculosis. Mol. Microbiol. 49:1493-1508. - PubMed
-
- Braibant, M., P. Gilot, and J. Content. 2000. The ATP binding cassette (ABC) transport systems of Mycobacterium tuberculosis. FEMS Microbiol. Rev. 24:449-467. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

