Toll-like receptor 4 mediates tolerance in macrophages stimulated with Toxoplasma gondii-derived heat shock protein 70
- PMID: 16040976
- PMCID: PMC1201250
- DOI: 10.1128/IAI.73.8.4634-4642.2005
Toll-like receptor 4 mediates tolerance in macrophages stimulated with Toxoplasma gondii-derived heat shock protein 70
Abstract
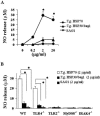
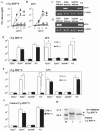
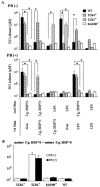
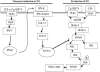
Peritoneal macrophages (PMs) from toll-like receptor 4 (TLR4)-deficient and wild-type (WT) mice were responsive to recombinant Toxoplasma gondii-derived heat shock protein 70 (rTgHSP70) and natural TgHSP70 (nTgHSP70) in NO release, but those from TLR2-, myeloid differentiation factor 88 (MyD88)-, and interleukin-1R-associated kinase 4 (IRAK4)-deficient mice were not. Polymyxin B did not inhibit PM activation by TgHSP70 and nTgHSP70 from WT and TLR4-deficient mice, while it inhibited PM activation by lipopolysaccharide. Pretreatment of PMs from WT but not from TLR4-deficient mice with rTgHSP70 resulted in suppression of NO release on restimulation with rTgHSP70. Similarly, pretreatment of PMs from WT but not TLR4-deficient mice with nTgHSP70 resulted in suppression of NO release on restimulation with nTgHSP70. Polymyxin B did not inhibit rTgHSP70- and nTgHSP70-induced tolerance of PMs from TLR4-deficient mice. Furthermore, PMs from WT mice increased suppressor of cytokine-signaling-1 (SOCS-1) expression after restimulation with rTgHSP70, while those from TLR4-deficient mice did not. Phosphorylation of JNK and I-kappaBalpha occurred in rTgHSP70-induced tolerance of PMs from TLR4-deficient mice, but not in that from WT mice. These data indicated that TgHSP70 signaling mechanisms were mediated by TLR2, MyD88, and IRAK4, but not by TLR4. On the other hand, signaling of TgHSP70-induced tolerance was mediated by TLR4, and the expression of SOCS-1 suppressed the TLR2 signaling pathway.
Figures



References
-
- Asea, A., M. Rehli, E. Kabingu, J. A. Boch, O. Bare, P. E. Auron, M. A. Stevenson, and S. K. Calderwood. 2002. Novel signal transduction pathway utilized by extracellular HSP70: role of toll-like receptor (TLR) 2 and TLR4. J. Biol. Chem. 277:15028-15034. - PubMed
-
- Beshay, E., F. Croze, and G. J. Prud'homme. 2001. The phosphodiesterase inhibitors pentoxifylline and rolipram suppress macrophage activation and nitric oxide production in vitro and in vivo. Clin. Immunol. 98:272-279. - PubMed
-
- Chen, C. C., K. T. Chiu, Y. T. Sun, and W. C. Chen. 1999. Role of the cyclic AMP-protein kinase a pathway in lipopolysaccharide-induced nitric oxide synthase expression in RAW 264.7 macrophages. Involvement of cyclooxygenase-2. J. Biol. Chem. 274:31559-31564. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

