Illustration of pneumococcal polysaccharide capsule during adherence and invasion of epithelial cells
- PMID: 16040978
- PMCID: PMC1201225
- DOI: 10.1128/IAI.73.8.4653-4667.2005
Illustration of pneumococcal polysaccharide capsule during adherence and invasion of epithelial cells
Abstract
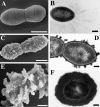
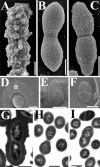
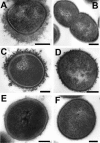
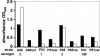
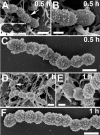
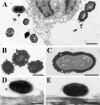
The capsular polysaccharide of Streptococcus pneumoniae represents an important virulence factor and protects against phagocytosis. In this study the amount of capsular polysaccharide present on the bacterial surface during the infection process was illustrated by electron microscopic studies. After infection of A549 cells (type II pneumocytes) and HEp-2 epithelial cells a modified fixation method was used that allowed visualization of the state of capsule expression. This modified fixation procedure did not require the use of capsule-specific antibodies. Visualization of pneumococci in intimate contact and invading cells demonstrated that pneumococci were devoid of capsular polysaccharide. Pneumococci not in contact with the cells did not show alterations in capsular polysaccharide. After infection of the cells, invasive pneumococci of different strains and serotypes were recovered. Single colonies of these recovered pneumococci exhibited an up-to-10(5)-fold-enhanced capacity to adhere and an up-to-10(4)-fold-enhanced capacity to invade epithelial cells. Electron microscopic studies using a lysine-ruthenium red (LRR) fixation procedure or cryo-field emission scanning electron microscopy revealed a reduction in capsular material, as determined in detail for a serotype 3 pneumococcal strain. The amount of polysaccharide in the serotype 3 capsule was also determined after intranasal infection of mice. This study illustrates for the first time the phenotypic variation of the polysaccharide capsule in the initial phase of pneumococcal infections. The modified LRR fixation allowed monitoring of the state of capsule expression of pathogens during the infectious process.
Figures



References
-
- Arrecubieta, C., E. Garcia, and R. Lopez. 1995. Sequence and transcriptional analysis of a DNA region involved in the production of capsular polysaccharide in Streptococcus pneumoniae type 3. Gene 167:1-7. - PubMed
-
- Austrian, R. 1981. Some observations on the pneumococcus and the current status of pneumococcal disease and its prevention. Rev. Infect. Dis. 3:S1-S17. - PubMed
-
- Austrian, R. 1986. Some aspects of the pneumococcal carrier state. J. Antimicrob. Chemother. 18(Suppl. A):35-45. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

