Protection by natural human immunoglobulin M antibody to meningococcal serogroup B capsular polysaccharide in the infant rat protection assay is independent of complement-mediated bacterial lysis
- PMID: 16040982
- PMCID: PMC1201264
- DOI: 10.1128/IAI.73.8.4694-4703.2005
Protection by natural human immunoglobulin M antibody to meningococcal serogroup B capsular polysaccharide in the infant rat protection assay is independent of complement-mediated bacterial lysis
Abstract
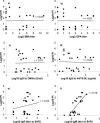
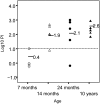
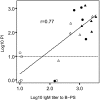
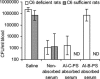
Neisseria meningitidis, an important cause of bacterial meningitis and septicemia worldwide, is associated with high mortality and serious sequelae. Natural immunity against meningococcal disease develops with age, but the specificity and functional activity of natural antibodies associated with protection are poorly understood. We addressed this question by using a selected subset of prevaccination sera (n = 26) with convergent or discrepant serum bactericidal activity (SBA) and infant rat protective activity (IRPA) against the serogroup B meningococcal strain 44/76-SL (B:15:P1.7,16) from Icelandic teenagers. The sera were analyzed by opsonophagocytic activity (OPA) assay, immunoblotting, immunoglobulin G (IgG) quantitation against live meningococcal cells by flow cytometry, and enzyme immunosorbent assay (EIA). High levels of SBA and OPA were reflected in distinct IgG binding to major outer membrane proteins and/or lipopolysaccharide in immunoblots. However, we could not detect any specific antibody patterns on blots that could explain IRPA. Only IgM antibody to group B capsular polysaccharide (B-PS), measured by EIA, correlated positively (r = 0.76, P < 0.001) with IRPA. Normal human sera (NHS; n = 20) from healthy Finnish children of different ages (7, 14, and 24 months and 10 years) supported this finding and showed an age-related increase in IRPA that coincided with the acquisition of B-PS specific IgM antibody. The protection was independent of complement-mediated bacterial lysis, as detected by the inability of NHS to augment SBA in the presence of human or infant rat complement and the equal protective activity of NHS in rat strains with fully functional or C6-deficient complement.
Figures


References
-
- Aase, A., G. Bjune, E. A. Høiby, E. Rosenqvist, A. K. Pedersen, and T. E. Michaelsen. 1995. Comparison among opsonic activity, antimeningococcal immunoglobulin G response, and serum bactericidal activity against meningococci in sera from vaccinees after immunization with a serogroup B outer membrane vesicle vaccine. Infect. Immun. 63:3531-3536. - PMC - PubMed
-
- Aase, A., E. A. Høiby, and T. E. Michaelsen. 1998. Opsonophagocytic and bactericidal activity mediated by purified IgG subclass antibodies after vaccination with the Norwegian group B meningococcal vaccine. Scand. J. Immunol. 47:388-396. - PubMed
-
- Artenstein, M. S., R. Gold, J. G. Zimmerly, F. A. Wyle, H. Schneider, and C. Harkins. 1970. Prevention of meningococcal disease by group C polysaccharide vaccine. N. Engl. J. Med. 282:417-420. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

