Conditions influencing the efficacy of vaccination with live organisms against Leishmania major infection
- PMID: 16040984
- PMCID: PMC1201197
- DOI: 10.1128/IAI.73.8.4714-4722.2005
Conditions influencing the efficacy of vaccination with live organisms against Leishmania major infection
Abstract
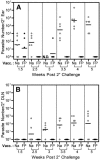
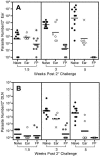
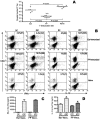
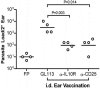
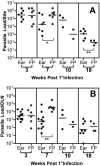
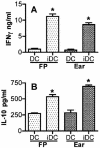
Numerous experimental vaccines have been developed with the goal of generating long-term cell-mediated immunity to the obligate intracellular parasite Leishmania major, yet inoculation with live, wild-type L. major remains the only successful vaccine in humans. We examined the expression of immunity at the site of secondary, low-dose challenge in the ear dermis to determine the kinetics of parasite clearance and the early events associated with the protection conferred by vaccination with live L. major organisms in C57BL/6 mice. Particular attention was given to the route of vaccination. We observed that the rapidity, strength, and durability of the memory response following subcutaneous vaccination with live parasites in the footpad are even greater than previously appreciated. Antigen-specific gamma interferon (IFN-gamma)-producing T cells infiltrate the secondary site by 1.5 weeks, and viable parasites are cleared as early as 2.5 weeks following rechallenge, followed by a rapid drop in IFN-gamma(+) CD4(+) cell numbers in the site. In comparison, intradermal vaccination with live parasites in the ear generates immunity that is delayed in effector cell recruitment to the rechallenge site and in the clearance of parasites from the site. This compromised immunity was associated with a rapid recruitment of interleukin-10 (IL-10)-producing CD4(+) T cells to the rechallenge site. Treatment with anti-IL-10-receptor or anti-CD25 antibody enhanced early parasite clearance in ear-vaccinated mice, indicating that chronic infection in the skin generates a population of regulatory cells capable of influencing the level of resistance to reinfection. A delicate balance of effector and regulatory T cells may be required to optimize the potency and durability of vaccines against Leishmaniasis and other intracellular pathogens.
Figures


References
-
- Alexander, J., G. H. Coombs, and J. C. Mottram. 1998. Leishmania mexicana cysteine proteinase-deficient mutants have attenuated virulence for mice and potentiate a Th1 response. J. Immunol. 161:6794-6801. - PubMed
-
- Asseman, C., S. Read, and F. Powrie. 2003. Colitogenic Th1 cells are present in the antigen-experienced T cell pool in normal mice: control by CD4+ regulatory T cells and IL-10. J. Immunol. 171:971-978. - PubMed
-
- Belkaid, Y., S. Kamhawi, G. Modi, J. Valenzuela, N. Noben-Trauth, E. Rowton, J. Ribeiro, and D. L. Sacks. 1998. Development of a natural model of cutaneous leishmaniasis: powerful effects of vector saliva and saliva preexposure on the long-term outcome of Leishmania major infection in the mouse ear dermis. J. Exp. Med. 188:1941-1953. - PMC - PubMed
-
- Belkaid, Y., S. Mendez, R. Lira, N. Kadambi, G. Milon, and D. Sacks. 2000. A natural model of Leishmania major infection reveals a prolonged “silent” phase of parasite amplification in the skin before the onset of lesion formation and immunity. J. Immunol. 165:969-977. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

