Characterization of the Helicobacter pylori cysteine-rich protein A as a T-helper cell type 1 polarizing agent
- PMID: 16040986
- PMCID: PMC1201243
- DOI: 10.1128/IAI.73.8.4732-4742.2005
Characterization of the Helicobacter pylori cysteine-rich protein A as a T-helper cell type 1 polarizing agent
Abstract
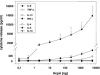
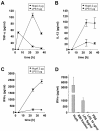
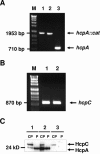
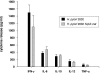
Predominant T-helper 1 (Th1) responses with increased gamma interferon (IFN-gamma) levels have been proposed to play an important role in Helicobacter pylori-induced gastritis and peptic ulceration. However, bacterial factors contributing to the initiation of Th1 polarization of H. pylori-specific immune responses have not been characterized in detail thus far. We report here on the identification of Helicobacter cysteine-rich protein A (HcpA) as a novel proinflammatory and Th1-promoting protein. The capacity of HcpA to induce immune activation was studied in splenocyte cultures of naive H. pylori-negative mice. HcpA stimulated the release of high concentrations of the proinflammatory and Th1-promoting cytokines interleukin-6 (IL-6) and IFN-gamma, in addition to significant levels of IL-12, tumor necrosis factor alpha, and IL-10. The observed cytokine profile was comparable to that induced by lipopolysaccharide but differed in the kinetics and maximum levels of cytokine production. In addition, HcpA-induced cytokine release resembled that observed upon incubation with H. pylori except for IL-10, which was only moderately released upon HcpA stimulation. Both HcpA- and H. pylori-mediated IFN-gamma production was drastically reduced by a neutralizing antibody against IL-12 but not by an anti-IL-2 antibody. Thus, HcpA seems to represent a novel bacterial virulence factor triggering the release of a concerted set of cytokines to instruct the adaptive immune system for the initiation of proinflammatory and Th1-biased immunity.
Figures



Similar articles
-
Modulation of the CD4+ T-cell response by Helicobacter pylori depends on known virulence factors and bacterial cholesterol and cholesterol α-glucoside content.J Infect Dis. 2011 Nov;204(9):1339-48. doi: 10.1093/infdis/jir547. Epub 2011 Sep 15. J Infect Dis. 2011. PMID: 21921201
-
Toll-like receptor 9 signaling has anti-inflammatory effects on the early phase of Helicobacter pylori-induced gastritis.Biochem Biophys Res Commun. 2012 Sep 28;426(3):342-9. doi: 10.1016/j.bbrc.2012.08.080. Epub 2012 Aug 24. Biochem Biophys Res Commun. 2012. PMID: 22940550
-
Mucosal immunization with a urease B DNA vaccine induces innate and cellular immune responses against Helicobacter pylori.Helicobacter. 2006 Apr;11(2):113-22. doi: 10.1111/j.1523-5378.2006.00385.x. Helicobacter. 2006. PMID: 16579841
-
The neutrophil-activating protein of Helicobacter pylori (HP-NAP) as an immune modulating agent.FEMS Immunol Med Microbiol. 2007 Jul;50(2):157-64. doi: 10.1111/j.1574-695X.2007.00258.x. Epub 2007 May 22. FEMS Immunol Med Microbiol. 2007. PMID: 17521355 Review.
-
Helicobacter pylori, asthma and allergy.FEMS Immunol Med Microbiol. 2009 Jun;56(1):1-8. doi: 10.1111/j.1574-695X.2009.00537.x. Epub 2009 Feb 10. FEMS Immunol Med Microbiol. 2009. PMID: 19220467 Review.
Cited by
-
Genomic erosion and extensive horizontal gene transfer in gut-associated Acetobacteraceae.BMC Genomics. 2019 Jun 10;20(1):472. doi: 10.1186/s12864-019-5844-5. BMC Genomics. 2019. PMID: 31182035 Free PMC article.
-
Core cysteine residues in the Plasminogen-Apple-Nematode (PAN) domain are critical for HGF/c-MET signaling.Commun Biol. 2022 Jul 1;5(1):646. doi: 10.1038/s42003-022-03582-8. Commun Biol. 2022. PMID: 35778602 Free PMC article.
-
The secreted Helicobacter cysteine-rich protein A causes adherence of human monocytes and differentiation into a macrophage-like phenotype.FEBS Lett. 2009 May 19;583(10):1637-43. doi: 10.1016/j.febslet.2009.04.027. Epub 2009 Apr 24. FEBS Lett. 2009. PMID: 19393649 Free PMC article.
-
Helicobacter pylori evolution: lineage- specific adaptations in homologs of eukaryotic Sel1-like genes.PLoS Comput Biol. 2007 Aug;3(8):e151. doi: 10.1371/journal.pcbi.0030151. Epub 2007 Jun 19. PLoS Comput Biol. 2007. PMID: 17696605 Free PMC article.
-
Structural and functional aspects of the Helicobacter pylori secretome.World J Gastroenterol. 2014 Feb 14;20(6):1402-23. doi: 10.3748/wjg.v20.i6.1402. World J Gastroenterol. 2014. PMID: 24587618 Free PMC article. Review.
References
-
- Aihara, M., K. Imagawa, Y. Funakoshi, Y. Ohmoto, and M. Kikuchi. 1998. Effects of rebamipide on production of several cytokines by human peripheral blood mononuclear cells. Dig. Dis. Sci. 43:160S-166S. - PubMed
-
- Atherton, J. C. 1998. Helicobacter pylori virulence factors. Br. Med. Bull. 54:105-120. - PubMed
-
- Atherton, J. C., R. M. Peek, K. T. Tham, T. L. Cover, and M. J. Blaser. 1997. Clinical and pathological importance of heterogeneity in vacA, the vacuolating cytotoxin gene of Helicobacter pylori. Gastroenterology 112:92-99. - PubMed
-
- Blaser, M. J. 1987. Gastric Campylobacter-like organisms, gastritis, and peptic ulcer disease. Gastroenterology 93:371-383. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

