Distinct defensin profiles in Neisseria gonorrhoeae and Chlamydia trachomatis urethritis reveal novel epithelial cell-neutrophil interactions
- PMID: 16040996
- PMCID: PMC1201278
- DOI: 10.1128/IAI.73.8.4823-4833.2005
Distinct defensin profiles in Neisseria gonorrhoeae and Chlamydia trachomatis urethritis reveal novel epithelial cell-neutrophil interactions
Abstract
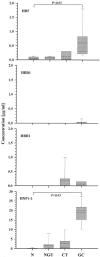
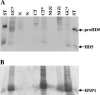
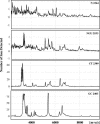
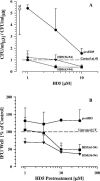
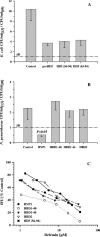
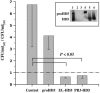
Defensins are key participants in mucosal innate defense. The varied antimicrobial activity and differential distribution of defensins at mucosal sites indicate that peptide repertoires are tailored to site-specific innate defense requirements. Nonetheless, few studies have investigated changes in peptide profiles and function after in vivo pathogen challenge. Here, we determined defensin profiles in urethral secretions of healthy men and men with Chlamydia trachomatis- and Neisseria gonorrhoeae-mediated urethritis by immunoblotting for the epithelial defensins HBD1, HBD2, and HD5 and the neutrophil defensins HNP1 to -3 (HNP1-3). HBD1 was not detectable in secretions, and HBD2 was only induced in a small proportion of the urethritis patients; however, HD5 and HNP1-3 were increased in C. trachomatis infection and significantly elevated in N. gonorrhoeae infection. When HNP1-3 levels were low, HD5 appeared mostly as the propeptide; however, when HNP1-3 levels were >10 microg/ml, HD5 was proteolytically processed, suggesting neutrophil proteases might contribute to HD5 processing. HD5 and HNP1-3 were bactericidal against C. trachomatis and N. gonorrhoeae, but HD5 activity was dependent upon N-terminal processing of the peptide. In vitro proteolysis of proHD5 by neutrophil proteases and analysis of urethral secretions by surface-enhanced laser desorption ionization substantiated that neutrophils contribute the key convertases for proHD5 in the urethra during these infections. This contrasts with the small intestine, where Paneth cells secrete both proHD5 and its processing enzyme, trypsin. In conclusion, we describe a unique defensin expression repertoire in response to inflammatory sexually transmitted infections and a novel host defense mechanism wherein epithelial cells collaborate with neutrophils to establish an antimicrobial barrier during infection.
Figures
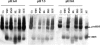
Similar articles
-
The prevalence of Chlamydia trachomatis and Neisseria gonorrhoeae infections among men with urethritis in Kuwait.J Infect Public Health. 2011 Sep;4(4):175-9. doi: 10.1016/j.jiph.2011.07.003. Epub 2011 Sep 3. J Infect Public Health. 2011. PMID: 22000844
-
Neisseria gonorrhoeae-induced human defensins 5 and 6 increase HIV infectivity: role in enhanced transmission.J Immunol. 2008 May 1;180(9):6176-85. doi: 10.4049/jimmunol.180.9.6176. J Immunol. 2008. PMID: 18424739 Free PMC article.
-
Human Defensins Inhibit SARS-CoV-2 Infection by Blocking Viral Entry.Viruses. 2021 Jun 26;13(7):1246. doi: 10.3390/v13071246. Viruses. 2021. PMID: 34206990 Free PMC article.
-
[The infections by Chlamydia trachomatis and Neisseria gonorrhoeae].Rinsho Byori. 2002 Nov;Suppl 123:57-61. Rinsho Byori. 2002. PMID: 12652791 Review. Japanese.
-
Diagnosis of Neisseria gonorrhoeae and Chlamydia trachomatis infections using antigen detection methods.Diagn Microbiol Infect Dis. 1986 Mar;4(3 Suppl):93S-99S. doi: 10.1016/s0732-8893(86)80047-5. Diagn Microbiol Infect Dis. 1986. PMID: 3084162 Review.
Cited by
-
DEFB126 2-nt Deletion (rs11467417) as a Potential Risk Factor for Chlamydia Trachomatis Infection and Subsequent Infertility in Iranian Men.J Reprod Infertil. 2024 Jan-Mar;25(1):20-27. doi: 10.18502/jri.v25i1.15195. J Reprod Infertil. 2024. PMID: 39157277 Free PMC article.
-
A microbial game of whack-a-mole: clinical case series of the urethral uncloaking phenomenon caused by Corynebacterium glucuronolyticum in men treated for Chlamydia trachomatis urethritis.Infection. 2019 Feb;47(1):121-124. doi: 10.1007/s15010-018-1211-8. Epub 2018 Aug 30. Infection. 2019. PMID: 30168068
-
Antimicrobial Peptides as a Promising Therapeutic Strategy for Neisseria Infections.Curr Microbiol. 2022 Feb 13;79(4):102. doi: 10.1007/s00284-022-02767-y. Curr Microbiol. 2022. PMID: 35152319 Review.
-
Sex hormone regulation of innate immunity in the female reproductive tract: the role of epithelial cells in balancing reproductive potential with protection against sexually transmitted pathogens.Am J Reprod Immunol. 2010 Jun;63(6):544-65. doi: 10.1111/j.1600-0897.2010.00842.x. Epub 2010 Mar 29. Am J Reprod Immunol. 2010. PMID: 20367623 Free PMC article.
-
HIV acquisition is associated with increased antimicrobial peptides and reduced HIV neutralizing IgA in the foreskin prepuce of uncircumcised men.PLoS Pathog. 2014 Oct 2;10(10):e1004416. doi: 10.1371/journal.ppat.1004416. eCollection 2014 Oct. PLoS Pathog. 2014. PMID: 25275513 Free PMC article. Clinical Trial.
References
-
- Avellar, M. C., L. Honda, K. G. Hamil, S. Yenugu, G. Grossman, P. Petrusz, F. S. French, and S. H. Hall. 2004. Differential expression and antibacterial activity of epididymis protein 2 isoforms in the male reproductive tract of human and rhesus monkey (Macaca mulatta). Biol. Reprod. 71:1453-1460. - PubMed
-
- Ayabe, T., D. P. Satchell, P. Pesendorfer, H. Tanabe, C. L. Wilson, S. J. Hagen, and A. J. Ouellette. 2002. Activation of Paneth cell alpha-defensins in mouse small intestine. J. Biol. Chem. 277:5219-5228. - PubMed
-
- Ayabe, T., D. P. Satchell, C. L. Wilson, W. C. Parks, M. E. Selsted, and A. J. Ouellette. 2000. Secretion of microbicidal alpha-defensins by intestinal Paneth cells in response to bacteria. Nat. Immunol. 1:113-118. - PubMed
-
- Bals, R., and P. S. Hiemstra. 2004. Innate immunity in the lung: how epithelial cells fight against respiratory pathogens. Eur. Respir. J. 23:327-333. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

