Low therapeutic threshold for hepatocyte replacement in murine phenylketonuria
- PMID: 16043102
- PMCID: PMC2694052
- DOI: 10.1016/j.ymthe.2005.03.025
Low therapeutic threshold for hepatocyte replacement in murine phenylketonuria
Abstract
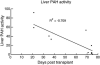
Phenylalanine homeostasis in mammals is primarily controlled by liver phenylalanine hydroxylase (PAH) activity. Inherited PAH deficiency (phenylketonuria or PKU) leads to hyperphenylalaninemia in both mice and humans. A low level of residual liver PAH activity ensures near-normal dietary protein tolerance with normal serum phenylalanine level, but the precise threshold for normal phenylalanine clearance is unknown. We employed hepatocyte transplantation under selective growth conditions to investigate the minimal number of PAH-expressing hepatocytes necessary to prevent hyperphenylalaninemia in mice. Serum phenylalanine levels remained normal in mice exhibiting nearly complete liver repopulation with PAH-deficient hepatocytes (<5% residual wild-type liver PAH activity). Conversely, transplantation of PAH-positive hepatocytes into PAH-deficient Pah(enu2) mice, a model of human PKU, yielded a significant decrease in serum phenylalanine (<700 muM) when liver repopulation exceeded approximately 5%. These data suggest that restoration of phenylalanine homeostasis requires PAH activity in only a minority of hepatocytes.
Figures



Similar articles
-
Hepatocytes from wild-type or heterozygous donors are equally effective in achieving successful therapeutic liver repopulation in murine phenylketonuria (PKU).Mol Genet Metab. 2011 Nov;104(3):235-40. doi: 10.1016/j.ymgme.2011.07.027. Epub 2011 Aug 4. Mol Genet Metab. 2011. PMID: 21917493 Free PMC article.
-
Expression of phenylalanine hydroxylase (PAH) in erythrogenic bone marrow does not correct hyperphenylalaninemia in Pah(enu2) mice.J Gene Med. 2003 Nov;5(11):984-93. doi: 10.1002/jgm.432. J Gene Med. 2003. PMID: 14601136 Free PMC article.
-
Complete correction of hyperphenylalaninemia following liver-directed, recombinant AAV2/8 vector-mediated gene therapy in murine phenylketonuria.Gene Ther. 2006 Mar;13(5):457-62. doi: 10.1038/sj.gt.3302678. Gene Ther. 2006. PMID: 16319949 Free PMC article.
-
Therapeutic liver repopulation for phenylketonuria.J Inherit Metab Dis. 2010 Dec;33(6):681-7. doi: 10.1007/s10545-010-9099-1. Epub 2010 May 22. J Inherit Metab Dis. 2010. PMID: 20495959 Review.
-
Mutations in the phenylalanine hydroxylase gene: genetic determinants for the phenotypic variability of hyperphenylalaninemia.Acta Paediatr Suppl. 1994 Dec;407:49-56. doi: 10.1111/j.1651-2227.1994.tb13451.x. Acta Paediatr Suppl. 1994. PMID: 7766959 Review.
Cited by
-
Treatment of phenylketonuria using minicircle-based naked-DNA gene transfer to murine liver.Hepatology. 2014 Sep;60(3):1035-43. doi: 10.1002/hep.27104. Epub 2014 Jul 29. Hepatology. 2014. PMID: 24585515 Free PMC article.
-
Blood phenylalanine reduction corrects CNS dopamine and serotonin deficiencies and partially improves behavioral performance in adult phenylketonuric mice.Mol Genet Metab. 2018 Jan;123(1):6-20. doi: 10.1016/j.ymgme.2017.10.009. Epub 2017 Oct 19. Mol Genet Metab. 2018. PMID: 29331172 Free PMC article.
-
Therapeutic hepatocyte transplant for inherited metabolic disorders: functional considerations, recent outcomes and future prospects.J Inherit Metab Dis. 2014 Mar;37(2):165-76. doi: 10.1007/s10545-013-9656-5. Epub 2013 Oct 2. J Inherit Metab Dis. 2014. PMID: 24085555 Free PMC article. Review.
-
State-of-the-Art 2019 on Gene Therapy for Phenylketonuria.Hum Gene Ther. 2019 Oct;30(10):1274-1283. doi: 10.1089/hum.2019.111. Epub 2019 Sep 9. Hum Gene Ther. 2019. PMID: 31364419 Free PMC article. Review.
-
A novel Pah-exon1 deleted murine model of phenylalanine hydroxylase (PAH) deficiency.Mol Genet Metab. 2020 Nov;131(3):306-315. doi: 10.1016/j.ymgme.2020.09.005. Epub 2020 Sep 30. Mol Genet Metab. 2020. PMID: 33051130 Free PMC article.
References
-
- Scriver CR, Kaufman S. Hyperphenylalaninemia: phenylalanine hydroxylase deficiency. In: Valle D, editor. The Metabolic & Molecular Bases of Inherited Disease. McGraw–Hill; New York: 2001. pp. 1667–1724.
-
- Udenfriend S, Cooper JR. The enzymatic conversion of phenylalanine to tyrosine. J. Biol. Chem. 1952;194:503–511. - PubMed
-
- Azen CG, et al. Intellectual development in 12-year-old children treated for phenylketonuria. Am. J. Dis. Child. 1991;145:35–39. - PubMed
-
- Fang B, et al. Gene therapy for phenylketonuria: phenotypic correction in a genetically deficient mouse model by adenovirus-mediated hepatic gene therapy. Gene Ther. 1994;1:247–254. - PubMed
-
- Laconi E, Laconi S. Principles of hepatocyte repopulation. Semin. Cell Dev. Biol. 2002;13:433–438. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

