Reversible intracellular translocation of KRas but not HRas in hippocampal neurons regulated by Ca2+/calmodulin
- PMID: 16043511
- PMCID: PMC2171478
- DOI: 10.1083/jcb.200409157
Reversible intracellular translocation of KRas but not HRas in hippocampal neurons regulated by Ca2+/calmodulin
Abstract
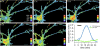
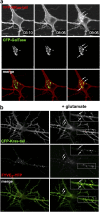
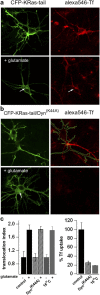
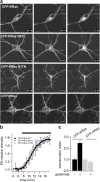
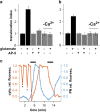
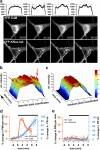
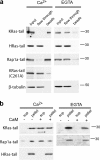
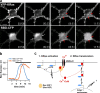
The Ras/MAPK pathway regulates synaptic plasticity and cell survival in neurons of the central nervous system. Here, we show that KRas, but not HRas, acutely translocates from the plasma membrane (PM) to the Golgi complex and early/recycling endosomes in response to neuronal activity. Translocation is reversible and mediated by the polybasic-prenyl membrane targeting motif of KRas. We provide evidence that KRas translocation occurs through sequestration of the polybasic-prenyl motif by Ca2+/calmodulin (Ca2+/CaM) and subsequent release of KRas from the PM, in a process reminiscent of GDP dissociation inhibitor-mediated membrane recycling of Rab and Rho GTPases. KRas translocation was accompanied by partial intracellular redistribution of its activity. We conclude that the polybasic-prenyl motif acts as a Ca2+/CaM-regulated molecular switch that controls PM concentration of KRas and redistributes its activity to internal sites. Our data thus define a novel signaling mechanism that differentially regulates KRas and HRas localization and activity in neurons.
Figures


References
-
- Aderem, A. 1992. The MARCKS brothers: a family of protein kinase C substrates. Cell. 71:713–716. - PubMed
-
- Ames, J.B., R. Ishima, T. Tanaka, J.I. Gordon, L. Stryer, and M. Ikura. 1997. Molecular mechanics of calcium-myristoyl switches. Nature. 389:198–202. - PubMed
-
- Arbuzova, A., J. Wang, D. Murray, J. Jacob, D.S. Cafiso, and S. McLaughlin. 1997. Kinetics of interaction of the myristoylated alanine-rich C kinase substrate, membranes, and calmodulin. J. Biol. Chem. 272:27167–27177. - PubMed
-
- Bijlmakers, M.J., and M. Marsh. 2003. The on-off story of protein palmitoylation. Trends Cell Biol. 13:32–42. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

