Self-assembled graphitic nanotubes with one-handed helical arrays of a chiral amphiphilic molecular graphene
- PMID: 16043721
- PMCID: PMC1182409
- DOI: 10.1073/pnas.0500852102
Self-assembled graphitic nanotubes with one-handed helical arrays of a chiral amphiphilic molecular graphene
Abstract
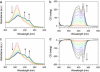
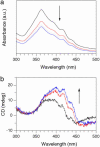
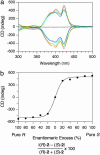
Self-assembly of a Gemini-shaped, chiral amphiphilic hexa-peri-hexabenzocoronene having two chiral oxyalkylene side chains, along with two lipophilic side chains, yields graphitic nanotubes with one-handed helical chirality. The nanotubes are characterized by an extremely high aspect ratio of >1,000 and have a uniform diameter of 20 nm and a wall thickness of 3 nm. The nanotubes with right- and left-handed helical senses were obtained from the (S)- and (R)-enantiomers of the amphiphile, respectively, due to an efficient translation of point chirality into supramolecular helical chirality. The (S)- and (R)-enantiomers coassemble at varying mole ratios to give nanotubes, whose circular dichroism profiles are almost unchanged over a wide range of the enantiomeric excess of the amphiphile (100-20%). The high level of chirality amplification thus observed indicates a long-range cooperativity in the self-assembling process. In sharp contrast, a hexabenzocoronene amphiphile with chiral lipophilic side chains did not form nanotubular assemblies. The present work demonstrates the majority rule in noncovalent systems and also may provide a synthetic strategy toward realization of molecular solenoids.
Figures





Similar articles
-
Self-assembled hexa-peri-hexabenzocoronene graphitic nanotube.Science. 2004 Jun 4;304(5676):1481-3. doi: 10.1126/science.1097789. Science. 2004. PMID: 15178796
-
Dynamic or nondynamic? Helical trajectory in hexabenzocoronene nanotubes biased by a detachable chiral auxiliary.J Am Chem Soc. 2013 Jan 9;135(1):114-7. doi: 10.1021/ja311738m. Epub 2012 Dec 26. J Am Chem Soc. 2013. PMID: 23252447
-
Hexabenzocoronene Graphitic Nanocoils Appended with Crown Ethers: Supramolecular Chirality Induced by Host-Guest Interaction.Chemistry. 2019 Dec 20;25(72):16692-16698. doi: 10.1002/chem.201904291. Epub 2019 Nov 21. Chemistry. 2019. PMID: 31591748
-
Helical Mesoporous Tantalum Oxide Nanotubes: Formation, Optical Activity, and Applications.Chem Rec. 2017 Nov;17(11):1146-1155. doi: 10.1002/tcr.201700012. Epub 2017 May 8. Chem Rec. 2017. PMID: 28480626 Review.
-
Hierarchy of Asymmetry in Chiral Supramolecular Polymers: Toward Functional, Helical Supramolecular Structures.Chemistry. 2019 Apr 23;25(23):5848-5864. doi: 10.1002/chem.201805577. Epub 2019 Feb 11. Chemistry. 2019. PMID: 30561853 Review.
Cited by
-
Molecular self-assembly into one-dimensional nanostructures.Acc Chem Res. 2008 Dec;41(12):1674-84. doi: 10.1021/ar8000926. Acc Chem Res. 2008. PMID: 18754628 Free PMC article.
-
Helix control in polymers: case of peptide nucleic acids (PNAs).Artif DNA PNA XNA. 2012 Apr-Jun;3(2):31-44. doi: 10.4161/adna.20572. Epub 2012 Apr 1. Artif DNA PNA XNA. 2012. PMID: 22772039 Free PMC article. Review.
-
Principles of self-assembly of helical pores from dendritic dipeptides.Proc Natl Acad Sci U S A. 2006 Feb 21;103(8):2518-23. doi: 10.1073/pnas.0509676103. Epub 2006 Feb 9. Proc Natl Acad Sci U S A. 2006. PMID: 16469843 Free PMC article.
-
Dynamic propeller conformation for the unprecedentedly high degree of chiral amplification of supramolecular helices.Chem Sci. 2016 Nov 1;7(11):6689-6694. doi: 10.1039/c6sc02814d. Epub 2016 Jul 12. Chem Sci. 2016. PMID: 28451111 Free PMC article.
-
Light-induced disassembly of self-assembled vesicle-capped nanotubes observed in real time.Nat Nanotechnol. 2011 Aug 14;6(9):547-52. doi: 10.1038/nnano.2011.120. Nat Nanotechnol. 2011. PMID: 21841795
References
-
- Green, M. M., Meijer, E. W. & Nolte, R. J. M., eds. (2003) Materials–Chirality, Topics in Stereochemistry (Wiley Interscience, Hoboken, NJ), Vol. 24.
-
- Hill, D. J., Mio, M. J., Prince, R. B., Hughes, T. S. & Moore, J. S. (2001) Chem. Rev. 101, 3893-4011. - PubMed
-
- Cornelissen, J. J. L. M., Rowan, A. E., Nolte, R. J. M. & Sommerdijk, N. A. J. M. (2001) Chem. Rev. 101, 4039-4070. - PubMed
-
- Nakano, T. & Okamoto, Y. (2001) Chem. Rev. 101, 4013-4038. - PubMed
-
- Green, M. M., Cheon, K.-S., Yang, S.-Y., Park, J.-W., Swansburg, S. & Liu, W. (2001) Acc. Chem. Res. 34, 672-680. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources

