Rapid increase in plasma membrane chloride permeability during wound resealing in starfish oocytes
- PMID: 16043775
- PMCID: PMC2266568
- DOI: 10.1085/jgp.200509294
Rapid increase in plasma membrane chloride permeability during wound resealing in starfish oocytes
Abstract
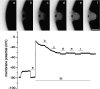
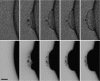
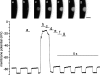
Plasma membrane wound repair is an important but poorly understood process. We used femtosecond pulses from a Ti-Sapphire laser to make multiphoton excitation-induced disruptions of the plasma membrane while monitoring the membrane potential and resistance. We observed two types of wounds that depolarized the plasma membrane. At threshold light levels, the membrane potential and resistance returned to prewound values within seconds; these wounds were not easily observed by light microscopy and resealed in the absence of extracellular Ca(2+). Higher light intensities create wounds that are easily visible by light microscopy and require extracellular Ca(2+) to reseal. Within a few seconds the membrane resistance is approximately 100-fold lower, while the membrane potential has depolarized from -80 to -30 mV and is now sensitive to the Cl(-) concentration but not to that of Na(+), K(+), or H(+). We suggest that the chloride sensitivity of the membrane potential, after wound resealing, is due to the fusion of chloride-permeable intracellular membranes with the plasma membrane.
Figures

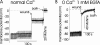


References
-
- Bansal, D., K. Miyake, S.S. Vogel, S. Groh, C.C. Chen, R. Williamson, P.L. McNeil, and K.P. Campbell. 2003. Defective membrane repair in dysferlin-deficient muscular dystrophy. Nature. 423:168–172. - PubMed
-
- Chambers, R. 1917. Microdissection studies. I. The visible structure of cell protoplasm and death changes. Am. J. Physiol. 43:1–12.
-
- Debska, G., A. Kicinska, J. Skalska, and A. Szewczyk. 2001. Intracellular potassium and chloride channels: an update. Acta Biochim. Pol. 48:137–144. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

