Cytomegalovirus MCK-2 controls mobilization and recruitment of myeloid progenitor cells to facilitate dissemination
- PMID: 16046529
- PMCID: PMC1895360
- DOI: 10.1182/blood-2005-05-1833
Cytomegalovirus MCK-2 controls mobilization and recruitment of myeloid progenitor cells to facilitate dissemination
Abstract
Murine cytomegalovirus encodes a secreted, pro-inflammatory chemokine-like protein, MCK-2, that recruits leukocytes and facilitates viral dissemination. We have shown that MCK-2-enhanced recruitment of myelomonocytic leukocytes with an immature phenotype occurs early during infection and is associated with efficient viral dissemination. Expression of MCK-2 drives the mobilization of a population of leukocytes from bone marrow that express myeloid marker Mac-1 (CD11b), intermediate levels of Gr-1 (Ly6 G/C), platelet-endothelial-cell adhesion molecule-1 (PECAM-1, CD31), together with heterogeneous levels of stem-cell antigen-1 (Sca-1, Ly-6 A /E). Recombinant MCK-2 mediates recruitment of this population even in the absence of viral infection. Recruitment of this cell population and viral dissemination via the bloodstream to salivary glands proceeds normally in mice that lack CCR2 and MCP-1 (CCL2), suggesting that recruitment of macrophages is not a requisite component of pathogenesis. Thus, a systemic impact of MCK-2 enhances the normal host response and causes a marked increase in myelomonocytic recruitment with an immature phenotype to initial sites of infection. Mobilization influences levels of virus dissemination via the bloodstream to salivary glands and is dependent on a myelomonocytic cell type other than mature macrophages.
Figures

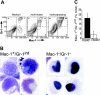




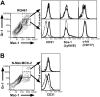

 ) mice following inoculation with 1 × 106 PFUs of mck-expressing RM427+. The mean and standard deviation (error bars) are indicated. (B) Viremia levels determined by ICA on groups of 3 mice at day 5 after inoculation (intraperitoneal route) with 105 PFUs of wild-type RQ461 in BALB/c and MCP-1-/- CCR2-/- mice. Mean (vertical line) and standard deviation (error bars) are indicated, along with data points from individual mice. (C) Titers of virus in spleen, lungs, and liver of RQ461 in BALB/c and MCP-1-/- CCR2-/- mice (same infection as panel A). (D) FCM analysis of Mac-1+/Gr-1int myeloid cells recovered from FP of MCP-1-/- CCR2-/- mice. FP leukocytes were prepared from groups of 3 mice at day 5 after inoculation with mck-expressing RM427+ or mck-mutant RM461 lacZ-tagged viruses (inoculum of 1 × 106 PFUs). FCM analysis and layout is described in the legend to Figure 2. (E) Day 14 virus titers in SGs following inoculation (FP route) with 107 PFUs of RQ461 (
) mice following inoculation with 1 × 106 PFUs of mck-expressing RM427+. The mean and standard deviation (error bars) are indicated. (B) Viremia levels determined by ICA on groups of 3 mice at day 5 after inoculation (intraperitoneal route) with 105 PFUs of wild-type RQ461 in BALB/c and MCP-1-/- CCR2-/- mice. Mean (vertical line) and standard deviation (error bars) are indicated, along with data points from individual mice. (C) Titers of virus in spleen, lungs, and liver of RQ461 in BALB/c and MCP-1-/- CCR2-/- mice (same infection as panel A). (D) FCM analysis of Mac-1+/Gr-1int myeloid cells recovered from FP of MCP-1-/- CCR2-/- mice. FP leukocytes were prepared from groups of 3 mice at day 5 after inoculation with mck-expressing RM427+ or mck-mutant RM461 lacZ-tagged viruses (inoculum of 1 × 106 PFUs). FCM analysis and layout is described in the legend to Figure 2. (E) Day 14 virus titers in SGs following inoculation (FP route) with 107 PFUs of RQ461 ( ) or RM461 (□).
) or RM461 (□).References
-
- Pass RF. Cytomegalovirus. In: Knipe DM, Howley PM, Griffin DE, Lamb RA, Martin MA, Roizman B, Straus SE, eds. Fields Virology. Vol 2. 4th ed. Philadelphia, PA: Lippincott Williams & Wilkins; 2001: 2675-2705.
-
- Saederup N, Mocarski ES. Fatal attraction: cytomegalovirus-encoded chemokine homologs. Curr Top Microbiol Immunol. 2002;269: 235-256. - PubMed
-
- Krmpotic A, Bubic I, Polic B, Lucin P, Jonjic S. Pathogenesis of murine cytomegalovirus infection. Microbes Infect. 2003;5: 1263-1277. - PubMed
-
- Mocarski ES Jr. Immune escape and exploitation strategies of cytomegaloviruses: impact on and imitation of the major histocompatibility system. Cell Microbiol. 2004;6: 707-717. - PubMed
-
- Gerna G, Zipeto D, Percivalle E, et al. Human cytomegalovirus infection of the major leukocyte subpopulations and evidence for initial viral replication in polymorphonuclear leukocytes from viremic patients. J Infect Dis. 1992;166: 1236-1244. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

