STAT3-mediated transcription of Bcl-2, Mcl-1 and c-IAP2 prevents apoptosis in polyamine-depleted cells
- PMID: 16048438
- PMCID: PMC1316269
- DOI: 10.1042/BJ20050465
STAT3-mediated transcription of Bcl-2, Mcl-1 and c-IAP2 prevents apoptosis in polyamine-depleted cells
Abstract
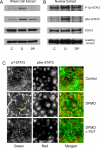
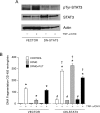
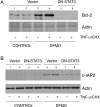
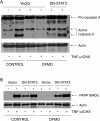
Activation of STAT3 (signal transducer and activator of transcription 3) plays a crucial role in cell survival and proliferation. The aim of the present study was to clarify the role of STAT3 signalling in the protection of polyamine-depleted intestinal epithelial cells against TNF-alpha (tumour necrosis factor-alpha)-induced apoptosis. Polyamine depletion by DFMO (alpha-difluoromethylornithine) caused phosphorylation of STAT3 at Tyr-705 and Ser-727. Phospho-Tyr-705 STAT3 was immunolocalized at the cell periphery and nucleus, whereas phospho-Ser-727 STAT3 was predominantly detected in the nucleus of polyamine-depleted cells. Sustained phosphorylation of STAT3 at tyrosine residues was observed in polyamine-depleted cells after exposure to TNF-alpha. Inhibition of STAT3 activation by AG490 or cell-membrane-permeant inhibitory peptide (PpYLKTK; where pY represents phospho-Tyr) increased the sensitivity of polyamine-depleted cells to apoptosis. Expression of DN-STAT3 (dominant negative-STAT3) completely eliminated the protective effect of DFMO against TNF-alpha-induced apoptosis. Polyamine depletion increased mRNA and protein levels for Bcl-2, Mcl-1 (myeloid cell leukaemia-1) and c-IAP2 (inhibitor of apoptosis protein-2). Significantly higher levels of Bcl-2 and c-IAP2 proteins were observed in polyamine-depleted cells before and after 9 h of TNF-alpha treatment. Inhibition of STAT3 by AG490 and DN-STAT3 decreased Bcl-2 promoter activity. DN-STAT3 decreased mRNA and protein levels for Bcl-2, Mcl-1 and c-IAP2 in polyamine-depleted cells. siRNA (small interfering RNA)-mediated inhibition of Bcl-2, Mcl-1 and c-IAP2 protein levels increased TNF-alpha-induced apoptosis. DN-STAT3 induced the activation of caspase-3 and PARP [poly(ADP-ribose) polymerase] cleavage in polyamine-depleted cells. These results suggest that activation of STAT3 in response to polyamine depletion increases the transcription and subsequent expression of anti-apoptotic Bcl-2 and IAP family proteins and thereby promotes survival of cells against TNF-alpha-induced apoptosis.
Figures





References
-
- Hall P. A., Coates P. J., Ansari B., Hopwood D. Regulation of cell number in the mammalian gastrointestinal tract–the importance of apoptosis. J. Cell. Sci. 1994;107:3569–3577. - PubMed
-
- Potten C. S. Epithelial cell growth and differentiation 2: Intestinal apoptosis. Am. J. Physiol. Gastrointest. Liver Physiol. 1997;273:G253–G257. - PubMed
-
- Schipper R. G., Penning L. C., Verhofstad A. A. J. Involvement of polyamines in apoptosis. Facts and controversies: effectors or protectors? Semin. Cancer Biol. Rev. 2000;2:55–68. - PubMed
-
- Ray R. M., Viar M. J., Yuan Q., Johnson L. R. Polyamine depletion delays apoptosis of rat intestinal epithelial cells. Am. J. Physiol. Cell Physiol. 2000;278:C480–C489. - PubMed
-
- Tabor C. W., Tabor H. Polyamines. Annu. Rev. Biochem. 1984;53:749–790. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

