Functional interrelationships between cell membrane and cell wall in antimicrobial peptide-mediated killing of Staphylococcus aureus
- PMID: 16048912
- PMCID: PMC1196293
- DOI: 10.1128/AAC.49.8.3114-3121.2005
Functional interrelationships between cell membrane and cell wall in antimicrobial peptide-mediated killing of Staphylococcus aureus
Abstract
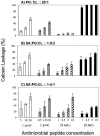
Perturbation of the Staphylococcus aureus cytoplasmic membrane (CM) is felt to play a key role in the microbicidal mechanism of many antimicrobial peptides (APs). However, it is not established whether membrane permeabilization (MP) alone is sufficient to kill susceptible staphylococci or if the cell wall (CW) and/or intracellular targets contribute to AP-induced lethality. We hypothesized that the relationships between MP and killing may differ for distinct APs. In this study, we investigated the association between AP-induced MP and lethality in S. aureus whole cells versus CW-free protoplasts, and in comparison to the MP of liposomes modeled after whole CMs in terms of phospholipid composition, fluidity and charge. Four APs with different structure-activity relationships were examined: thrombin-induced platelet microbicidal protein 1 (tPMP-1), human neutrophil protein 1 (hNP-1), gramicidin D, and polymyxin B. MP was quantified fluorometrically by calcein release. All APs tested, except polymyxin B, caused concentration-dependent MP and killing of whole cells, but not of protoplasts. The reduced AP susceptibility of protoplasts was associated with increased cardiolipin and lysyl-phosphatidylglycerol content and reduced fluidity of their CMs. However, liposomal MP induced by tPMP-1, hNP-1, and gramicidin D paralleled that of whole cells. Collectively, these results indicate that (i) structurally distinct APs likely exert their staphylocidal effects by differing mechanisms, (ii) MP is not the sole event leading to AP-induced staphylocidal activity, (iii) a complex interrelationship exists between the CM and CW in AP-induced killing, and (iv) liposomes modeled upon whole cell or protoplast CMs can recapitulate the respective susceptibilities to killing by distinct APs.
Figures
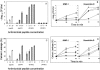
 ), gramicidin D (
), gramicidin D (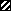 ), and polymyxin B (▪) were used in this study. Each experiment was done in triplicate, and the values represent means ± standard deviations. (B) Kinetics of staphylocidal activity. Killing of S. aureus over time following exposure to tPMP-1 at 0.5 μg/ml (•), 1.0 μg/ml (▪), or 2.0 μg/ml (▴) and gramicidin D at 1× MIC (•), 2.5× MIC (▪), 5× MIC (▴), or 10× MIC (
), and polymyxin B (▪) were used in this study. Each experiment was done in triplicate, and the values represent means ± standard deviations. (B) Kinetics of staphylocidal activity. Killing of S. aureus over time following exposure to tPMP-1 at 0.5 μg/ml (•), 1.0 μg/ml (▪), or 2.0 μg/ml (▴) and gramicidin D at 1× MIC (•), 2.5× MIC (▪), 5× MIC (▴), or 10× MIC ( ). (C) S. aureus whole-cell membrane permeabilization by antimicrobial peptides. Membrane permeabilization of S. aureus whole cells by antimicrobial peptides for 1-h exposure was detected via the leakage of calcein. hNP-1 (□), tPMP-1 (
). (C) S. aureus whole-cell membrane permeabilization by antimicrobial peptides. Membrane permeabilization of S. aureus whole cells by antimicrobial peptides for 1-h exposure was detected via the leakage of calcein. hNP-1 (□), tPMP-1 ( ), gramicidin D (
), gramicidin D (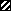 ), and polymyxin B (▪) were used in this study. Each experiment was done in triplicate, and the values represent means ± standard deviations. (D) Kinetics of membrane permeabilization. Membrane permeabilization of S. aureus over time following exposure to tPMP-1 at 0.5 μg/ml (•), 1.0 μg/ml (▪), or 2.0 μg/ml (▴) and gramicidin D at 1× MIC (•), 2.5× MIC (▪), 5× MIC (▴), or 10× MIC (
), and polymyxin B (▪) were used in this study. Each experiment was done in triplicate, and the values represent means ± standard deviations. (D) Kinetics of membrane permeabilization. Membrane permeabilization of S. aureus over time following exposure to tPMP-1 at 0.5 μg/ml (•), 1.0 μg/ml (▪), or 2.0 μg/ml (▴) and gramicidin D at 1× MIC (•), 2.5× MIC (▪), 5× MIC (▴), or 10× MIC ( ). (E) Correlation analysis of the relationship between killing (−Δlog10 CFU/ml) and membrane permeabilization (% calcein leakage). Linear regressions were determined, and correlation coefficients (r2) were calculated from mean results of three independent experiments. r2 for tPMP-1 (⧫), hNP-1 (□), and gramicidin D (▵) are 0.9925, 0.9961, and 0.8074, respectively (P < 0.05 for each).
). (E) Correlation analysis of the relationship between killing (−Δlog10 CFU/ml) and membrane permeabilization (% calcein leakage). Linear regressions were determined, and correlation coefficients (r2) were calculated from mean results of three independent experiments. r2 for tPMP-1 (⧫), hNP-1 (□), and gramicidin D (▵) are 0.9925, 0.9961, and 0.8074, respectively (P < 0.05 for each).
 ), gramicidin D (
), gramicidin D (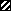 ), and polymyxin B (▪) were used in this study. Each experiment was done in triplicate, and the values represent means ± standard deviations. (B) Kinetics of staphylocidal activity. Killing of S. aureus over time following exposure to tPMP-1 at 0.5 μg/ml (•), 1.0 μg/ml (▪), or 2.0 μg/ml (▴) and gramicidin D at 1× MIC (•), 2.5× MIC (▪), 5× MIC (▴), or 10× MIC (
), and polymyxin B (▪) were used in this study. Each experiment was done in triplicate, and the values represent means ± standard deviations. (B) Kinetics of staphylocidal activity. Killing of S. aureus over time following exposure to tPMP-1 at 0.5 μg/ml (•), 1.0 μg/ml (▪), or 2.0 μg/ml (▴) and gramicidin D at 1× MIC (•), 2.5× MIC (▪), 5× MIC (▴), or 10× MIC ( ). (C) S. aureus whole-cell membrane permeabilization by antimicrobial peptides. Membrane permeabilization of S. aureus whole cells by antimicrobial peptides for 1-h exposure was detected via the leakage of calcein. hNP-1 (□), tPMP-1 (
). (C) S. aureus whole-cell membrane permeabilization by antimicrobial peptides. Membrane permeabilization of S. aureus whole cells by antimicrobial peptides for 1-h exposure was detected via the leakage of calcein. hNP-1 (□), tPMP-1 ( ), gramicidin D (
), gramicidin D ( ), and polymyxin B (▪) were used in this study. Each experiment was done in triplicate, and the values represent means ± standard deviations. (D) Kinetics of membrane permeabilization. Membrane permeabilization of S. aureus over time following exposure to tPMP-1 at 0.5 μg/ml (•), 1.0 μg/ml (▪), or 2.0 μg/ml (▴) and gramicidin D at 1× MIC (•), 2.5× MIC (▪), 5× MIC (▴), or 10× MIC (
), and polymyxin B (▪) were used in this study. Each experiment was done in triplicate, and the values represent means ± standard deviations. (D) Kinetics of membrane permeabilization. Membrane permeabilization of S. aureus over time following exposure to tPMP-1 at 0.5 μg/ml (•), 1.0 μg/ml (▪), or 2.0 μg/ml (▴) and gramicidin D at 1× MIC (•), 2.5× MIC (▪), 5× MIC (▴), or 10× MIC ( ). (E) Correlation analysis of the relationship between killing (−Δlog10 CFU/ml) and membrane permeabilization (% calcein leakage). Linear regressions were determined, and correlation coefficients (r2) were calculated from mean results of three independent experiments. r2 for tPMP-1 (⧫), hNP-1 (□), and gramicidin D (▵) are 0.9925, 0.9961, and 0.8074, respectively (P < 0.05 for each).
). (E) Correlation analysis of the relationship between killing (−Δlog10 CFU/ml) and membrane permeabilization (% calcein leakage). Linear regressions were determined, and correlation coefficients (r2) were calculated from mean results of three independent experiments. r2 for tPMP-1 (⧫), hNP-1 (□), and gramicidin D (▵) are 0.9925, 0.9961, and 0.8074, respectively (P < 0.05 for each).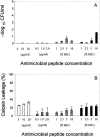
 ), gramicidin D (
), gramicidin D ( ), and polymyxin B (▪) were used in this study. Each experiment was done in triplicate, and the values represent means ± standard deviations.
), and polymyxin B (▪) were used in this study. Each experiment was done in triplicate, and the values represent means ± standard deviations.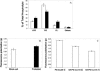
 ), gramicidin D (
), gramicidin D ( ), and polymyxin B (▪) were used in this study. Each experiment was done in triplicate, and the values represent means ± SDs.
), and polymyxin B (▪) were used in this study. Each experiment was done in triplicate, and the values represent means ± SDs.Similar articles
-
Diversity in antistaphylococcal mechanisms among membrane-targeting antimicrobial peptides.Infect Immun. 2001 Aug;69(8):4916-22. doi: 10.1128/IAI.69.8.4916-4922.2001. Infect Immun. 2001. PMID: 11447168 Free PMC article.
-
In vitro antibacterial activities of platelet microbicidal protein and neutrophil defensin against Staphylococcus aureus are influenced by antibiotics differing in mechanism of action.Antimicrob Agents Chemother. 1999 May;43(5):1111-7. doi: 10.1128/AAC.43.5.1111. Antimicrob Agents Chemother. 1999. PMID: 10223922 Free PMC article.
-
A synthetic congener modeled on a microbicidal domain of thrombin- induced platelet microbicidal protein 1 recapitulates staphylocidal mechanisms of the native molecule.Antimicrob Agents Chemother. 2006 Nov;50(11):3786-92. doi: 10.1128/AAC.00038-06. Epub 2006 Sep 5. Antimicrob Agents Chemother. 2006. PMID: 16954324 Free PMC article.
-
Platelet microbicidal proteins and neutrophil defensin disrupt the Staphylococcus aureus cytoplasmic membrane by distinct mechanisms of action.J Clin Invest. 1998 Jan 1;101(1):178-87. doi: 10.1172/JCI562. J Clin Invest. 1998. PMID: 9421480 Free PMC article.
-
[Structural and functional characteristics of gramicidin S in connection with its antibiotic activity].Antibiot Khimioter. 2003;48(12):29-32. Antibiot Khimioter. 2003. PMID: 15176102 Review. Russian. No abstract available.
Cited by
-
Ceftaroline increases membrane binding and enhances the activity of daptomycin against daptomycin-nonsusceptible vancomycin-intermediate Staphylococcus aureus in a pharmacokinetic/pharmacodynamic model.Antimicrob Agents Chemother. 2013 Jan;57(1):66-73. doi: 10.1128/AAC.01586-12. Epub 2012 Oct 15. Antimicrob Agents Chemother. 2013. PMID: 23070161 Free PMC article.
-
Alternative mutational pathways to intermediate resistance to vancomycin in methicillin-resistant Staphylococcus aureus.J Infect Dis. 2013 Jul;208(1):67-74. doi: 10.1093/infdis/jit127. Epub 2013 Mar 28. J Infect Dis. 2013. PMID: 23539745 Free PMC article.
-
Failures in clinical treatment of Staphylococcus aureus Infection with daptomycin are associated with alterations in surface charge, membrane phospholipid asymmetry, and drug binding.Antimicrob Agents Chemother. 2008 Jan;52(1):269-78. doi: 10.1128/AAC.00719-07. Epub 2007 Oct 22. Antimicrob Agents Chemother. 2008. PMID: 17954690 Free PMC article.
-
Lipidated Short Analogue of α-Melanocyte Stimulating Hormone Exerts Bactericidal Activity against the Stationary Phase of Methicillin-Resistant Staphylococcus aureus and Inhibits Biofilm Formation.ACS Omega. 2020 Oct 26;5(44):28425-28440. doi: 10.1021/acsomega.0c01462. eCollection 2020 Nov 10. ACS Omega. 2020. PMID: 33195893 Free PMC article.
-
Enhanced expression of dltABCD is associated with the development of daptomycin nonsusceptibility in a clinical endocarditis isolate of Staphylococcus aureus.J Infect Dis. 2009 Dec 15;200(12):1916-20. doi: 10.1086/648473. J Infect Dis. 2009. PMID: 19919306 Free PMC article.
References
-
- Bayer, A. S., R. P. Prasad, J. Chandra, A. Koul, M. Smriti, A. Varma, R. A. Skurray, N. Firth, M. H. Brown, S.-P. Koo, and M. R. Yeaman. 2000. In vitro resistance of Staphylococcus aureus to thrombin-induced platelet microbicidal protein in associated with alterations in cytoplasmic membrane fluidity. Infect. Immun. 68:3548-3553. - PMC - PubMed
-
- Burkhart, B. M., R. M. Gassman, D. A. Langs, W. A. Pangborn, W. L. Duax, and V. Pletnev. 1999. Gramicidin D conformation, dynamics and membrane ion transport. Biopolymers 51:129-144. - PubMed
-
- Chen, C.-C. H., and D. S. Feingold. 1973. The mechanism of polymyxin B action and selectivity towards biologic membranes. Biochemistry 12:2105-2111. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

