Lipophilic antifolate trimetrexate is a potent inhibitor of Trypanosoma cruzi: prospect for chemotherapy of Chagas' disease
- PMID: 16048931
- PMCID: PMC1196212
- DOI: 10.1128/AAC.49.8.3234-3238.2005
Lipophilic antifolate trimetrexate is a potent inhibitor of Trypanosoma cruzi: prospect for chemotherapy of Chagas' disease
Abstract
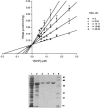
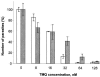
Trypanosoma cruzi, a protozoan parasite, is the causative agent for Chagas' disease, which poses serious public health problem in Latin America. The two drugs available for the treatment of this disease are effective only against recent infections and are toxic. Dihydrofolate reductase (DHFR) has a proven track record as a drug target. The lipophilic antifolate trimetrexate (TMQ), which is an FDA-approved drug for the treatment of Pneumocystis carinii infection in AIDS patients, is a potent inhibitor of T. cruzi DHFR activity, with an inhibitory constant of 6.6 nM. The compound is also highly effective in killing T. cruzi parasites. The 50 and 90% lethal dose values against the trypomastigote are 19 and 36 nM, and the corresponding values for the amastigote form are 26 and 72 nM, respectively. However, as TMQ is also a good inhibitor of human DHFR, further improvement of the selectivity of this drug would be preferable. Identification of a novel antifolate selective against T. cruzi would open up new therapeutic avenues for treatment of Chagas' disease.
Figures
Similar articles
-
Structures of dihydrofolate reductase-thymidylate synthase of Trypanosoma cruzi in the folate-free state and in complex with two antifolate drugs, trimetrexate and methotrexate.Acta Crystallogr D Biol Crystallogr. 2009 Jul;65(Pt 7):704-16. doi: 10.1107/S090744490901230X. Epub 2009 Jun 20. Acta Crystallogr D Biol Crystallogr. 2009. PMID: 19564691
-
Synthesis and characterization of potent inhibitors of Trypanosoma cruzi dihydrofolate reductase.Bioorg Med Chem. 2010 Jun 1;18(11):4056-66. doi: 10.1016/j.bmc.2010.04.020. Epub 2010 Apr 9. Bioorg Med Chem. 2010. PMID: 20452776
-
Lipophilic antifolates as agents against opportunistic infections. 1. Agents superior to trimetrexate and piritrexim against Toxoplasma gondii and Pneumocystis carinii in in vitro evaluations.J Med Chem. 1996 Mar 15;39(6):1271-80. doi: 10.1021/jm950760y. J Med Chem. 1996. PMID: 8632434
-
[The chemotherapy of Chagas disease].Medicina (B Aires). 1999;59 Suppl 2:147-65. Medicina (B Aires). 1999. PMID: 10668258 Review. Spanish.
-
Inhibitors of dihydrofolate reductase in Leishmania and trypanosomes.Biochim Biophys Acta. 2002 Jul 18;1587(2-3):249-57. doi: 10.1016/s0925-4439(02)00088-1. Biochim Biophys Acta. 2002. PMID: 12084467 Review.
Cited by
-
Preclinical evaluation of the antifolate QN254, 5-chloro- N'6'-(2,5-dimethoxy-benzyl)-quinazoline-2,4,6-triamine, as an antimalarial drug candidate.Antimicrob Agents Chemother. 2010 Jun;54(6):2603-10. doi: 10.1128/AAC.01526-09. Epub 2010 Mar 29. Antimicrob Agents Chemother. 2010. PMID: 20350951 Free PMC article.
-
Chemical and genetic validation of dihydrofolate reductase-thymidylate synthase as a drug target in African trypanosomes.Mol Microbiol. 2008 Jul;69(2):520-33. doi: 10.1111/j.1365-2958.2008.06305.x. Mol Microbiol. 2008. PMID: 18557814 Free PMC article.
-
Targeting one-carbon metabolism for cancer immunotherapy.Clin Transl Med. 2024 Jan;14(1):e1521. doi: 10.1002/ctm2.1521. Clin Transl Med. 2024. PMID: 38279895 Free PMC article. Review.
-
Repositioned Drugs for Chagas Disease Unveiled via Structure-Based Drug Repositioning.Int J Mol Sci. 2020 Nov 20;21(22):8809. doi: 10.3390/ijms21228809. Int J Mol Sci. 2020. PMID: 33233837 Free PMC article.
-
The Trypanosoma cruzi TcrNT2 Nucleoside Transporter Is a Conduit for the Uptake of 5-F-2'-Deoxyuridine and Tubercidin Analogues.Molecules. 2022 Nov 19;27(22):0. doi: 10.3390/molecules27228045. Molecules. 2022. PMID: 36432150 Free PMC article.
References
-
- Beck, J. T., and B. Ullman. 1990. Nutritional requirements of wild-type and folate transport deficient Leishmania donovani for pterins and folates. Mol. Biochem. Parasitol. 43:221-230. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

