Mammary serine protease inhibitor (Maspin) binds directly to interferon regulatory factor 6: identification of a novel serpin partnership
- PMID: 16049006
- PMCID: PMC3175759
- DOI: 10.1074/jbc.M503523200
Mammary serine protease inhibitor (Maspin) binds directly to interferon regulatory factor 6: identification of a novel serpin partnership
Abstract
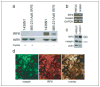
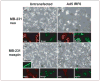
Since its reported discovery in 1994, maspin (mammary serine protease inhibitor) has been characterized as a class II tumor suppressor by its ability to promote apoptosis and inhibit cell invasion. Maspin is highly expressed in normal mammary epithelial cells but reduced or absent in aggressive breast carcinomas. However, despite efforts to characterize the mechanism(s) by which maspin functions as a tumor suppressor, its molecular characterization has remained somewhat elusive. Therefore, in an attempt to identify maspin-interacting proteins and thereby gain insight into the functional pathways of maspin, we employed a maspin-baited yeast two-hybrid system and subsequently identified Interferon Regulatory Factor 6 (IRF6) as a maspin-binding protein. IRF6 belongs to the IRF family of transcription factors, which is best known for its regulation of interferon and interferon-inducible genes following a pathogenic stimulus. Although many of the IRF family members have been well characterized, IRF6 remains poorly understood. We report that IRF6 is expressed in normal mammary epithelial cells and that it directly associates with maspin in a yeast two-hybrid system and in vitro. The interaction occurs via the conserved IRF protein association domain and is regulated by phosphorylation of IRF6. We have shown that, similar to maspin, IRF6 expression is inversely correlated with breast cancer invasiveness. We further demonstrated that the transient re-expression of IRF6 in breast cancer cells results in an increase of N-cadherin and a redistribution of vimentin commensurate with changes in cell morphology, suggestive of an epithelial-to-mesenchymal transition event. Concomitantly, we showed that maspin acts as a negative regulator of this process. These findings help to elucidate the molecular mechanisms of maspin and suggest an interactive role between maspin and IRF6 in regulating cellular phenotype, the loss of which can lead to neoplastic transformation.
Figures





References
-
- Zou Z, Anisowicz A, Hendrix MJC, Thor A, Neveu M, Sheng S, Rafidi K, Seftor E, Sager R. Science. 1994;263:526–529. - PubMed
-
- Zhang M, Volpert O, Shi YH, Bouck N. Nat Med. 2000;6:196–199. - PubMed
-
- Jiang N, Meng Y, Zhang S, Mensah-Osman E, Sheng S. Oncogene. 2002;21:4089–4098. - PubMed
-
- Zhang M, Magit D, Botteri F, Shi HY, He K, Li M, Furth P, Sager R. Dev Biol. 1999;215:278–287. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

