Emergent patterns of growth controlled by multicellular form and mechanics
- PMID: 16049098
- PMCID: PMC1187971
- DOI: 10.1073/pnas.0502575102
Emergent patterns of growth controlled by multicellular form and mechanics
Abstract
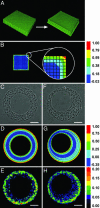
Spatial patterns of cellular growth generate mechanical stresses that help to push, fold, expand, and deform tissues into their specific forms. Genetic factors are thought to specify patterns of growth and other behaviors to drive morphogenesis. Here, we show that tissue form itself can feed back to regulate patterns of proliferation. Using micro-fabrication to control the organization of sheets of cells, we demonstrated the emergence of stable patterns of proliferative foci. Regions of concentrated growth corresponded to regions of high tractional stress generated within the sheet, as predicted by a finite-element model of multicellular mechanics and measured directly by using a micromechanical force sensor array. Inhibiting actomyosin-based tension or cadherin-mediated connections between cells disrupted the spatial pattern of proliferation. These findings demonstrate the existence of patterns of mechanical forces that originate from the contraction of cells, emerge from their multicellular organization, and result in patterns of growth. Thus, tissue form is not only a consequence but also an active regulator of tissue growth.
Figures



Comment in
-
Mechanical control of tissue growth: function follows form.Proc Natl Acad Sci U S A. 2005 Aug 16;102(33):11571-2. doi: 10.1073/pnas.0505939102. Epub 2005 Aug 9. Proc Natl Acad Sci U S A. 2005. PMID: 16091458 Free PMC article. No abstract available.
References
-
- Thompson, D. A. W. (1917) On Growth and Form (Cambridge Univ. Press, Cambridge, U.K.).
-
- Salazar-Ciudad, I., Jernvall, J. & Newman, S. A. (2003) Development (Cambridge, U.K.) 130, 2027–2037. - PubMed
-
- Steinberg, M. S. (1962) Science 137, 762–763. - PubMed
-
- Keller, R. E. (1980) J. Embryol. Exp. Morphol. 60, 201–234. - PubMed
-
- Schoenwolf, G. C. & Franks, M. V. (1984) Dev. Biol. 105, 257–272. - PubMed

