Interaction via a key tryptophan in the I-II linker of N-type calcium channels is required for beta1 but not for palmitoylated beta2, implicating an additional binding site in the regulation of channel voltage-dependent properties
- PMID: 16049174
- PMCID: PMC6724838
- DOI: 10.1523/JNEUROSCI.1137-05.2005
Interaction via a key tryptophan in the I-II linker of N-type calcium channels is required for beta1 but not for palmitoylated beta2, implicating an additional binding site in the regulation of channel voltage-dependent properties
Erratum in
- J Neurosci. 2005 Oct 5;25(40):table of contents. Richards, Mark S [corrected to Richards, Mark W]
Abstract
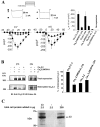
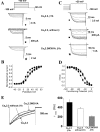
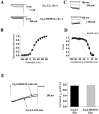
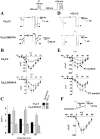
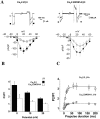
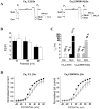
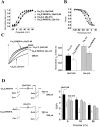
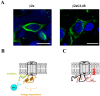
The CaVbeta subunits of voltage-gated calcium channels regulate these channels in several ways. Here we investigate the role of these auxiliary subunits in the expression of functional N-type channels at the plasma membrane and in the modulation by G-protein-coupled receptors of this neuronal channel. To do so, we mutated tryptophan 391 to an alanine within the alpha-interacting domain (AID) in the I-II linker of CaV2.2. We showed that the mutation W391 virtually abolishes the binding of CaVbeta1b and CaVbeta2a to the CaV2.2 I-II linker and strongly reduced current density and cell surface expression of both CaV2.2/alpha2delta-2/beta1b and/beta2a channels. When associated with CaVbeta1b, the W391A mutation also prevented the CaVbeta1b-mediated hyperpolarization of CaV2.2 channel activation and steady-state inactivation. However, the mutated CaV2.2W391A/beta1b channels were still inhibited to a similar extent by activation of the D2 dopamine receptor with the agonist quinpirole. Nevertheless, key hallmarks of G-protein modulation of N-type currents, such as slowed activation kinetics and prepulse facilitation, were not observed for the mutated channel. In contrast, CaVbeta2a was still able to completely modulate the biophysical properties of CaV2.2W391A channel and allow voltage-dependent G-protein modulation of CaV2.2W391A. Additional data suggest that the concentration of CaVbeta2a in the proximity of the channel is enhanced independently of its binding to the AID by its palmitoylation. This is essentially sufficient for all of the functional effects of CaVbeta2a, which may occur via a second lower-affinity binding site, except trafficking the channel to the plasma membrane, which requires interaction with the AID region.
Figures


Similar articles
-
The importance of occupancy rather than affinity of CaV(beta) subunits for the calcium channel I-II linker in relation to calcium channel function.J Physiol. 2006 Jul 15;574(Pt 2):387-98. doi: 10.1113/jphysiol.2006.109744. Epub 2006 Apr 20. J Physiol. 2006. PMID: 16627564 Free PMC article.
-
Inhibition of protein kinase C (PKC) response of voltage-gated calcium (Cav)2.2 channels expressed in Xenopus oocytes by Cavβ subunits.Neuroscience. 2014 Nov 7;280:1-9. doi: 10.1016/j.neuroscience.2014.08.049. Epub 2014 Sep 9. Neuroscience. 2014. PMID: 25218964
-
Translocatable voltage-gated Ca2+ channel β subunits in α1-β complexes reveal competitive replacement yet no spontaneous dissociation.Proc Natl Acad Sci U S A. 2018 Oct 16;115(42):E9934-E9943. doi: 10.1073/pnas.1809762115. Epub 2018 Sep 26. Proc Natl Acad Sci U S A. 2018. PMID: 30257950 Free PMC article.
-
Calcium channel auxiliary α2δ and β subunits: trafficking and one step beyond.Nat Rev Neurosci. 2012 Jul 18;13(8):542-55. doi: 10.1038/nrn3311. Nat Rev Neurosci. 2012. PMID: 22805911 Review.
-
Voltage-gated calcium channel α 2δ subunits: an assessment of proposed novel roles.F1000Res. 2018 Nov 21;7:F1000 Faculty Rev-1830. doi: 10.12688/f1000research.16104.1. eCollection 2018. F1000Res. 2018. PMID: 30519455 Free PMC article. Review.
Cited by
-
Ankyrin-B regulates Cav2.1 and Cav2.2 channel expression and targeting.J Biol Chem. 2014 Feb 21;289(8):5285-95. doi: 10.1074/jbc.M113.523639. Epub 2014 Jan 6. J Biol Chem. 2014. PMID: 24394417 Free PMC article.
-
Constitutive activity of the Ghrelin receptor reduces surface expression of voltage-gated Ca2+ channels in a CaVβ-dependent manner.J Cell Sci. 2017 Nov 15;130(22):3907-3917. doi: 10.1242/jcs.207886. Epub 2017 Oct 16. J Cell Sci. 2017. PMID: 29038230 Free PMC article.
-
Dose-dependent and isoform-specific modulation of Ca2+ channels by RGK GTPases.J Gen Physiol. 2006 Nov;128(5):605-13. doi: 10.1085/jgp.200609631. J Gen Physiol. 2006. PMID: 17074979 Free PMC article.
-
The life cycle of voltage-gated Ca2+ channels in neurons: an update on the trafficking of neuronal calcium channels.Neuronal Signal. 2021 Feb 23;5(1):NS20200095. doi: 10.1042/NS20200095. eCollection 2021 Apr. Neuronal Signal. 2021. PMID: 33664982 Free PMC article. Review.
-
Calmodulin-dependent gating of Ca(v)1.2 calcium channels in the absence of Ca(v)beta subunits.Proc Natl Acad Sci U S A. 2008 Jun 10;105(23):8154-9. doi: 10.1073/pnas.0711624105. Epub 2008 Jun 5. Proc Natl Acad Sci U S A. 2008. PMID: 18535142 Free PMC article.
References
-
- Altier C, Dubel SJ, Barrere C, Jarvis SE, Stotz SC, Spaetgens RL, Scott JD, Cornet V, De Waard M, Zamponi GW, Nargeot J, Bourinet E (2002) Trafficking of L-type calcium channels mediated by the postsynaptic scaffolding protein AKAP79. J Biol Chem 277: 33598-33603. - PubMed
-
- Barclay J, Balaguero N, Mione M, Ackerman SL, Letts VA, Brodbeck J, Canti C, Meir A, Page KM, Kusumi K, Perez-Reyes E, Lander ES, Frankel WN, Gardiner RM, Dolphin AC, Rees M (2001) Ducky mouse phenotype of epilepsy and ataxia is associated with mutations in the Cacna2d2 gene and decreased calcium channel current in cerebellar Purkinje cells. J Neurosci 21: 6095-6104. - PMC - PubMed
-
- Bean BP (1989) Neurotransmitter inhibition of neuronal calcium currents by changes in channel voltage dependence. Nature 340: 153-156. - PubMed
-
- Bell DC, Butcher AJ, Berrow NS, Page KM, Brust PF, Nesterova A, Stauderman KA, Seabrook GR, Nurnberg B, Dolphin AC (2001) Biophysical properties, pharmacology, and modulation of human, neuronal L-type (α1D, CaV1.3) voltage-dependent calcium currents. J Neurophysiol 85: 816-827. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
