Pax6 is required for making specific subpopulations of granule and periglomerular neurons in the olfactory bulb
- PMID: 16049175
- PMCID: PMC6724841
- DOI: 10.1523/JNEUROSCI.1435-05.2005
Pax6 is required for making specific subpopulations of granule and periglomerular neurons in the olfactory bulb
Abstract
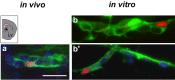
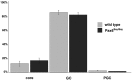
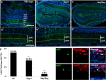
The subventricular zone (SVZ) produces different subclasses of olfactory bulb (OB) interneurons throughout life. Little is known about the molecular mechanisms controlling the production of different types of interneurons. Here we show that most proliferating adult SVZ progenitors express the transcription factor Pax6, but only a small subpopulation of migrating neuroblasts and new OB interneurons derived from these progenitors retains Pax6 expression. To elucidate the cell-autonomous role of Pax6 in OB neurogenesis, we transplanted green fluorescent protein-expressing embryonic forebrain progenitors of the dorsal lateral ganglionic eminence from Pax6 mutant Small Eye (Pax6(Sey/Sey)) mice into the SVZ of adult wild-type mice. Pax6(Sey/Sey) progenitors produce neuroblasts capable of migrating into the OB but fail to generate dopaminergic periglomerular and superficial granule cells. Interestingly, superficial granule neurons also express mRNA for tyrosine hydroxylase, the rate-limiting enzyme in dopamine synthesis. Our data show that SVZ neuroblasts are heterogeneous and that Pax6 is required in a cell-autonomous manner for the production of cells in the dopaminergic lineage.
Figures



Similar articles
-
Ventralized dorsal telencephalic progenitors in Pax6 mutant mice generate GABA interneurons of a lateral ganglionic eminence fate.Proc Natl Acad Sci U S A. 2005 May 17;102(20):7374-9. doi: 10.1073/pnas.0500819102. Epub 2005 May 6. Proc Natl Acad Sci U S A. 2005. PMID: 15878992 Free PMC article.
-
Meis2 is a Pax6 co-factor in neurogenesis and dopaminergic periglomerular fate specification in the adult olfactory bulb.Development. 2014 Jan;141(1):28-38. doi: 10.1242/dev.097295. Epub 2013 Nov 27. Development. 2014. PMID: 24284204
-
A dlx2- and pax6-dependent transcriptional code for periglomerular neuron specification in the adult olfactory bulb.J Neurosci. 2008 Jun 18;28(25):6439-52. doi: 10.1523/JNEUROSCI.0700-08.2008. J Neurosci. 2008. PMID: 18562615 Free PMC article.
-
Role of a transcription factor Pax6 in the developing vertebrate olfactory system.Dev Growth Differ. 2007 Dec;49(9):683-90. doi: 10.1111/j.1440-169X.2007.00965.x. Epub 2007 Oct 1. Dev Growth Differ. 2007. PMID: 17908181 Review.
-
Concise review: Pax6 transcription factor contributes to both embryonic and adult neurogenesis as a multifunctional regulator.Stem Cells. 2008 Jul;26(7):1663-72. doi: 10.1634/stemcells.2007-0884. Epub 2008 May 8. Stem Cells. 2008. PMID: 18467663 Review.
Cited by
-
The Impact of Mitochondrial Dysfunction on Dopaminergic Neurons in the Olfactory Bulb and Odor Detection.Mol Neurobiol. 2020 Sep;57(9):3646-3657. doi: 10.1007/s12035-020-01947-w. Epub 2020 Jun 20. Mol Neurobiol. 2020. PMID: 32564285 Free PMC article.
-
Dlx-dependent and -independent regulation of olfactory bulb interneuron differentiation.J Neurosci. 2007 Mar 21;27(12):3230-43. doi: 10.1523/JNEUROSCI.5265-06.2007. J Neurosci. 2007. PMID: 17376983 Free PMC article.
-
Dopaminergic Neurones in the Main Olfactory Bulb: An Overview from an Electrophysiological Perspective.Front Neuroanat. 2017 Feb 14;11:7. doi: 10.3389/fnana.2017.00007. eCollection 2017. Front Neuroanat. 2017. PMID: 28261065 Free PMC article. Review.
-
FGF-Dependent, Context-Driven Role for FRS Adapters in the Early Telencephalon.J Neurosci. 2017 Jun 7;37(23):5690-5698. doi: 10.1523/JNEUROSCI.2931-16.2017. Epub 2017 May 8. J Neurosci. 2017. PMID: 28483978 Free PMC article.
-
High spatial resolution gene expression profiling and characterization of neuroblasts migrating in the peri-injured cortex using photo-isolation chemistry.Front Neurosci. 2025 Jan 7;18:1504047. doi: 10.3389/fnins.2024.1504047. eCollection 2024. Front Neurosci. 2025. PMID: 39840011 Free PMC article.
References
-
- Anderson S, Mione M, Yun K, Rubenstein JL (1999) Differential origins of neocortical projection and local circuit neurons: role of dlx genes in neocortical interneuronogenesis. Cereb Cortex 9: 646-654. - PubMed
-
- Baker H (1990) Unilateral, neonatal olfactory deprivation alters tyrosine hydroxylase expression but not aromatic amino acid decarboxylase or GABA immunoreactivity. Neuroscience 36: 761-771. - PubMed
-
- Brown JP, Couillard-Despres S, Cooper-Kuhn CM, Winkler J, Aigner L, Kuhn HG (2003) Transient expression of doublecortin during adult neurogenesis. J Comp Neurol 467: 1-10. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous
