An optical multifrequency phase-modulation method using microbeads for measuring intracellular oxygen concentrations in plants
- PMID: 16049223
- PMCID: PMC1366618
- DOI: 10.1529/biophysj.105.063453
An optical multifrequency phase-modulation method using microbeads for measuring intracellular oxygen concentrations in plants
Abstract
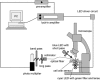
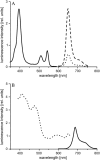
A technique has been developed to measure absolute intracellular oxygen concentrations in green plants. Oxygen-sensitive phosphorescent microbeads were injected into the cells and an optical multifrequency phase-modulation technique was used to discriminate the sensor signal from the strong autofluorescence of the plant tissue. The method was established using photosynthesis-competent cells of the giant algae Chara corallina L., and was validated by application to various cell types of other plant species.
Figures

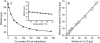


Similar articles
-
Feasibility of diffuse optical imaging with long-lived luminescent probes.Opt Lett. 2006 Apr 15;31(8):1082-4. doi: 10.1364/ol.31.001082. Opt Lett. 2006. PMID: 16625910 Free PMC article.
-
Intracellular axial current in Chara corallina reflects the altered kinetics of ions in cytoplasm under the influence of light.Biophys J. 2005 Jan;88(1):690-7. doi: 10.1529/biophysj.104.044974. Epub 2004 Oct 29. Biophys J. 2005. PMID: 15516526 Free PMC article.
-
Combined depth- and time-resolved autofluorescence spectroscopy of epithelial tissue.Opt Lett. 2006 Jun 15;31(12):1833-5. doi: 10.1364/ol.31.001833. Opt Lett. 2006. PMID: 16729086
-
Optical oxygen micro- and nanosensors for plant applications.Sensors (Basel). 2012;12(6):7015-32. doi: 10.3390/s120607015. Epub 2012 May 25. Sensors (Basel). 2012. PMID: 22969334 Free PMC article. Review.
-
Cell and molecular biology of the fastest myosins.Int Rev Cell Mol Biol. 2009;276:301-47. doi: 10.1016/S1937-6448(09)76007-1. Int Rev Cell Mol Biol. 2009. PMID: 19584016 Review.
Cited by
-
Noninvasive Oxygen Monitoring in Three-Dimensional Tissue Cultures Under Static and Dynamic Culture Conditions.Biores Open Access. 2015 May 1;4(1):266-77. doi: 10.1089/biores.2015.0004. eCollection 2015. Biores Open Access. 2015. PMID: 26309802 Free PMC article.
-
Nanoparticle PEBBLE sensors in live cells and in vivo.Annu Rev Anal Chem (Palo Alto Calif). 2009;2:57-76. doi: 10.1146/annurev.anchem.1.031207.112823. Annu Rev Anal Chem (Palo Alto Calif). 2009. PMID: 20098636 Free PMC article. Review.
-
Oxygen Sensor-Based Respirometry and the Landscape of Microbial Testing Methods as Applicable to Food and Beverage Matrices.Sensors (Basel). 2023 May 6;23(9):4519. doi: 10.3390/s23094519. Sensors (Basel). 2023. PMID: 37177723 Free PMC article. Review.
-
Measuring ROS and redox markers in plant cells.RSC Chem Biol. 2021 Jun 29;2(5):1384-1401. doi: 10.1039/d1cb00071c. eCollection 2021 Oct 7. RSC Chem Biol. 2021. PMID: 34704044 Free PMC article. Review.
-
Distributed fiber optical sensing of oxygen with optical time domain reflectometry.Sensors (Basel). 2013 May 31;13(6):7170-83. doi: 10.3390/s130607170. Sensors (Basel). 2013. PMID: 23727953 Free PMC article.
References
-
- Geigenberger, P., A. R. Fernie, Y. Gibon, M. Christ, and M. Stitt. 2000. Metabolic activity decreases as an adaptive response to low internal oxygen in growing potato tubers. Biol. Chem. 381:723–740. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

