Clinical research subject recruitment: the Volunteer for Vanderbilt Research Program www.volunteer.mc.vanderbilt.edu
- PMID: 16049231
- PMCID: PMC1294031
- DOI: 10.1197/jamia.M1722
Clinical research subject recruitment: the Volunteer for Vanderbilt Research Program www.volunteer.mc.vanderbilt.edu
Abstract
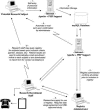
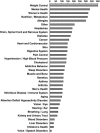
This article provides information concerning a novel research subject recruitment registry developed at Vanderbilt University. Project goals were (1) to provide a mechanism for lay individuals to self-enter information conveying interest in volunteering for clinical research and (2) provide tools for researchers to select and contact potential volunteers based on study-specific inclusion criteria. The registry was built and offered as an institutional resource to all university scientists conducting institutional review board-approved research. The authors present (1) a model for redesigning workflow associated with subject registration, volunteer retrieval, and subject contact; (2) details of a Web-based software application used as a focal point in designing workflow for our system; (3) descriptive statistics for volunteer and researcher use of the system during the first 32 months of operation; (4) cost estimates for the project; and (5) a set of recommendations for other medical centers wishing to adopt similar methodology.
Figures
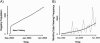
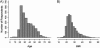
References
-
- National Heart, Lung, and Blood Institute. Terms and conditions for accrual of research subjects in research. Available from: http://www.nhlbi.nih.gov/funding/policies/terms.htm.
-
- Marks RG, Conlon M, Ruberg SJ. Paradigm shifts in clinical trials enabled by information technology. Stat Med. 2001;20:2683–96. - PubMed
-
- Marks L, Power E. Using technology to address recruitment issues in the clinical trial process. Trends Biotechnol. 2002;20:105–9. - PubMed
-
- PHP Hypertext Preprocessor. Available from: http://www.php.net/.
-
- mySQL Database Engine. Available from: http://www.mysql.com/.
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

