Glomerular aging in females is a multi-stage reversible process mediated by phenotypic changes in progenitors
- PMID: 16049323
- PMCID: PMC1603557
- DOI: 10.1016/S0002-9440(10)62981-1
Glomerular aging in females is a multi-stage reversible process mediated by phenotypic changes in progenitors
Abstract
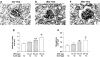
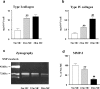
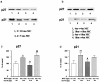
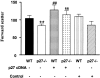
The glomeruli of postmenopausal C57BL6 mice, and age-matched males, show progressive hypertrophy and glomerulosclerosis. We asked whether this was a multistage process, was due to alterations in glomerular progenitors, and was reversible in female mice. Using cross bone marrow transplants (BMT) between young and old females, we found that BMT delivered a phenotype that was donor age-specific. The fact that lesions in young recipients were more severe if the donors were in late rather than early menopause suggested that new progenitor phenotypes had appeared. Postmenopausal recipients of BMT from young donors had reduced glomerular hypertrophy and sclerosis, implying that the aging lesions in females were reversible and that progenitors, rather than the local environment, determined the glomerular profile. The altered phenotype included increased extracellular matrix synthesis and decreased matrix metalloproteinase-2 levels as well as cell hypertrophy. The mechanism of the cellular hypertrophy was due to uncoupling of hypertrophy from proliferation, resulting from elevated p27 levels. Thus, glomerular hypertrophy and sclerosis in aging females is a multistage process, is reversible, and may be determined by the phenotype of bone marrow-derived progenitor cells.
Figures


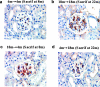
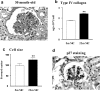
Similar articles
-
Glomerulosclerosis is transmitted by bone marrow-derived mesangial cell progenitors.J Clin Invest. 2001 Dec;108(11):1649-56. doi: 10.1172/JCI12916. J Clin Invest. 2001. PMID: 11733560 Free PMC article.
-
Mechanical stretch induces podocyte hypertrophy in vitro.Kidney Int. 2005 Jan;67(1):157-66. doi: 10.1111/j.1523-1755.2005.00066.x. Kidney Int. 2005. PMID: 15610239
-
Overexpression of alpha1beta1 integrin directly affects rat mesangial cell behavior.Kidney Int. 2000 Sep;58(3):1088-97. doi: 10.1046/j.1523-1755.2000.00266.x. Kidney Int. 2000. PMID: 10972673
-
Mechanical stress reduces podocyte proliferation in vitro.Kidney Int. 2002 Jan;61(1):40-50. doi: 10.1046/j.1523-1755.2002.00102.x. Kidney Int. 2002. PMID: 11786083
-
Role of mesangium in microcirculatory control.Blood Purif. 1997;15(4-6):228-31. doi: 10.1159/000170340. Blood Purif. 1997. PMID: 9435950 Review. No abstract available.
Cited by
-
The role of renin angiotensin system inhibition in kidney repair.Fibrogenesis Tissue Repair. 2010 May 4;3:7. doi: 10.1186/1755-1536-3-7. Fibrogenesis Tissue Repair. 2010. PMID: 20441574 Free PMC article.
-
Low-protein diet supplemented with ketoacids reduces the severity of renal disease in 5/6 nephrectomized rats: a role for KLF15.Kidney Int. 2011 May;79(9):987-96. doi: 10.1038/ki.2010.539. Epub 2011 Jan 19. Kidney Int. 2011. PMID: 21248717 Free PMC article.
-
Cells derived from young bone marrow alleviate renal aging.J Am Soc Nephrol. 2011 Nov;22(11):2028-36. doi: 10.1681/ASN.2010090982. Epub 2011 Sep 30. J Am Soc Nephrol. 2011. PMID: 21965376 Free PMC article.
-
Age dependent accumulation patterns of advanced glycation end product receptor (RAGE) ligands and binding intensities between RAGE and its ligands differ in the liver, kidney, and skeletal muscle.Immun Ageing. 2017 Jun 5;14:12. doi: 10.1186/s12979-017-0095-2. eCollection 2017. Immun Ageing. 2017. PMID: 28592983 Free PMC article.
-
Reduced oxidant stress and extended lifespan in mice exposed to a low glycotoxin diet: association with increased AGER1 expression.Am J Pathol. 2007 Jun;170(6):1893-902. doi: 10.2353/ajpath.2007.061281. Am J Pathol. 2007. PMID: 17525257 Free PMC article.
References
-
- Coresh J, Astor BC, Greene T, Eknoyan G, Levey AS. Prevalence of chronic kidney disease and decreased kidney function in the adult US population: Third National Health and Nutrition Examination Survey. Am J Kidney Dis. 2003;41:1–12. - PubMed
-
- US Renal Data System Washington, DC: The National Institutes of Health, NIDDK; USRDS 1999 Annual Report. 1999
-
- Zheng F, Cornaccia F, Schulman I, Banerjee A, Cheng QL, Potier M, Plati AR, Berho M, Elliot SJ, Li J, Fornoni A, Zang YJ, Zisman A, Striker LJ, Striker GE. Development of albuminuria and glomerular lesions in normoglycemic B6 recipients of db/db mice bone marrow: the role of mesangial cell progenitors. Diabetes. 2004;53:2420–2427. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

